Epidemiology of carbapenem resistant Enterobacteriaceae (CRE) during 2000-2012 in Asia
Introduction
Enterobacteriaceae family is an important cause of urinary tract infections (UTIs), bloodstream infections, hospital acquired pneumonias and various intra-abdominal infections. The prevalence of ESBLs producing Enterobacteriaceae made carbapenem, a broad-spectrum antimicrobial agent became a preferred drug in the treatment of multi-drug-resistant (MDR) Enterobacteriaceae. Because of their safety and established efficacy, carbapenems used to serve as the ultimate last resort option for treating MDR. Over the past decade, the emergence of carbapenem resistant Enterobacteriaceae (CRE) has become a formidable threat to public health. The prevalence of CRE, according to some institutions in epidemic area, varies between 24.7% and 29.8% (1,2). The rapid and extensive dissemination of CRE demonstrated that we still lacked sufficient and effective measures to reverse or at least control the current situation.
The emergence of CRE is a menace to patients, particularly to those who are debilitated, with various underlying diseases, complex infections or medical interventions (3). Moreover, pathogens resistance to carbapenems often shows high resistance to other antibiotic agents as well, such as cephalosporins, quinolones and aminoglycosides, leaving few or, in some cases, no optimal therapeutic options. What’s more, some extra factors, such as delayed identification, lack of accurate judgment of pathogens, also lead to high mortality, prolonged hospital stay, and huge medical expenses in CRE infected patients (4,5).
To hinder the spread of CRE, it’s important for us to know its epidemiology first. Recently, many Asian countries have reported the emergence of CRE as well as the rates of resistance (6-8). Based on the data published during 2000-2013, this article aims to give a comprehensive analysis of the prevalence of carbapenem resistance in Enterobacteriaceae across Asian area. Information documented in this study may offer significant help in the control and empirical antibiotic treatment of CRE.
Methods
Search strategy
A systematic literature search has been conducted of PubMed and Embase (for articles published from January 2001 to December 2013). Our search strategy uses the following terms: “carbapenem” “imipenem” “meropenem” “Enterobacteriaceae” “E. coli” “Klebsiella” “Enterobacter” “Serratia” “Citrobacter” “Morganella” “Asia”. The search is not limited by language.
Study selection
Two authors (Yanling Xu and Bing Gu) have independently performed the literature search following such criteria: (I) basic experimental studies and reviews are not eligible for inclusion; (II) studies that only offered rates of susceptibility or focused on selected isolates with certain drug resistance pattern or genes are not included either; (III) studies on clinical or animal research were also excluded; (IV) according to our literature search, no data was reported about the prevalence of CRE in the year of 2013. Therefore we divided the previous 13 years [2000-2012] into three periods (2000-2004, 2005-2008, and 2009-2012). Studies that could not be allocated into any of the three periods will not be included. All discrepancies between the two reviewers were resolved by the consensus of all authors.
We also assess the adherence to standard of experimental procedures in each article, including details of if the susceptibility test was in accordance with guidelines established by the Clinical and Laboratory Standards Institute (CLSI); if the reports provide the exact study years and countries/regions; when data in one study was overlapped with another, the data in the more recent and larger studies was included in the analysis. Data from these smaller studies were used if additional useful information could be extracted.
Data extraction
Two reviewers independently extracted the relevant data, using a standardized collection form to extract data from the included articles. The following data gets extracted from each study: (I) years that the bacteria were isolated; (II) countries; (III) bacteria species; (IV) number of the whole tested strains; (V) carbapenems tested (only imipenem and meropenem were included in this study due to the lack of relevant data of other carbapenems); (VI) number of resistance strains or the rates of resistance to carbapenem.
If an integer is not obtained when doing multiplication between the number of whole strains and the resistance rate, the rounding method is used.
Outcomes analysis
The heterogeneity between studies is assessed by using the Q-statistic test; a P value <0.1 defines statistical significance in the analysis of heterogeneity. We present results from the fixed effects model when there is no heterogeneity between the analyzed studies (P>0.1); otherwise, we present results from the random effects model. Freeman-Tukey arcsine transformations are used to stabilize variances. We perform data manipulation and statistical analyses by statistical software package STATA 10.0 (STATACorp, College Station, TX, USA).
Results
Result of the systematic literature search
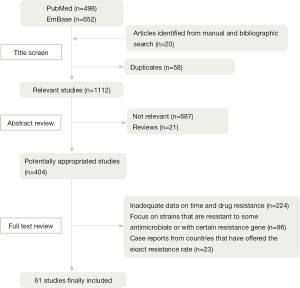
Figure 1 shows a flow diagram describing our selection process, which is applied to identify the eligible studies. Among 1,150 articles found through electronic and reference list searches, 61 studies met the inclusion criteria and are included in this meta-analysis.
Pathogen distribution in Asia
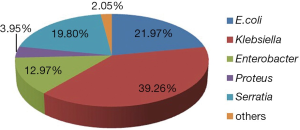
Among the Enterobacteriaceae genera, the most commonly organisms collected in this study are E. coli, Klebsiella spp., Enterobacte spp., Serratia spp., Proteus spp. and Citrobacter spp. Only case reports are obtained on the carbapenem resistance in Morganella spp. and Shigella spp. As Figure 2 shows, Klebsiella spp. and E. coli account for the largest proportion of CRE, namely 39.3% and 22.0%, and then followed by Serratia spp. (19.8%), Enterobacter spp. (13.0%), Proteus spp. (4.0%), and Citribacter spp. (2.0%).
Overall carbapenem resistance in Enterobacteriaceae in Asia
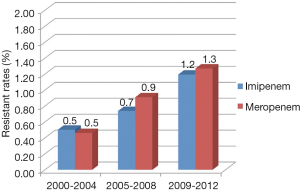
During the period of 2001 to 2012, the rates of resistance to impenem and meropenem in Enterobacteriaceae are 0.8% (95% CI, 0.6-0.9%) and 1.0% (95% CI, 0.8-1.3%) respectively. Though the resistant rates are still low, they keep an escalating trend from 2000 to 2012. Figure 3 explicitly depicts the prevalence of CRE during each study period. In the first period [2000-2003], the Enterobacteriaceae resistance rates of imipenem and meropenem are both 0.5%, and the rates increased stably afterwards. From 2009 to 2012, the resistance rates rise to 1.2% (95% CI, 0.9-1.5%) and 1.3% (95% CI, 1.0-1.7%). No obvious difference is observed between the resistance rates of imipenem and meropenem.
CRE in different Asia countries
For all the 49 Asian countries (or regions), only 37.5% [19] of them contribute epidemiology data of CRE, and 18 of them provide exact resistance rates during the study years [2000-2012]. The rest ones provide either only case reports (Israel, Oman, Bangladesh and so on) (9-13) or no information at all (Figure 4).
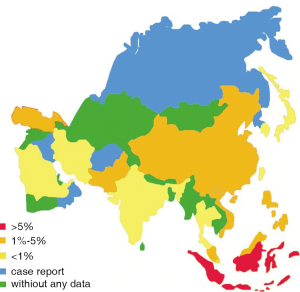
Among all the Enterobacteriaceae collected, the rates of resistance to imipenem vary from 0.1% (95% CI, 0.1-1.2%) to 5.8% (95% CI, 2.2-11.0%) with different Asian countries. The top three countries with the highest resistance rates to imipenem are Indonesia (5.8%), Vietnam (3.0%) and Philippines (3.7%). Singapore (0.1%), Kuwait (0.1%) and Japan (0.2%) show the lowest resistance rates. Rate of resistance to imipenem in China is 1.4% (95% CI, 1.0-1.6%), higher than the average rate (0.7%).
As to meropenem resistance in Enterobacteriaceae, only six countries contribute available data. The average rate of resistance to meropenem is 0.9% (95% CI, 0.7-1.2%). Turkey (2.9%) and India (2.6%) show higher resistance rates than others. In China, the prevalence of meropenem resistance rate among Enterobacteriaceae is 1.4% (95% CI, 1.0-1.9%).
CRE in different species
Table 1 demonstrates the resistance pattern of impenem and meropenem in each genus of Enterobacteriaceae from 2000 to 2012. The rank order of imipenem resistance in Enterobacteriaceae is as follows: Serratia spp. > Proteu spp. > Klebsiella spp. > Enterobacter spp. = Citrobacter spp. > E. coli. The rates of resistance to meropenem in each genus generally follow the same order as imipenem.
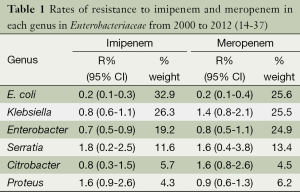
Full table
Three most commonly isolated Enterobacteriaceae genera, Klebsiella, E. coli and Enterobacter, are analyzed to discover their resistance trends to carbapenems (imipenem and meropenem) during the collection years (Table 2).

Full table
Among the three most common Enterobacteriaceae genera, Klebsiella spp. has the highest resistance rates to imipenem and meropenem. In 2000-2004, its resistance rates are 0.5% (95% CI, 0.2-0.8%) and 0.3% (95% CI, 0.-0.8%), and they keep stably escalating trends during the later years. In 2009-2011, they has risen to 1.9% (95% CI, 1.3-2.7%) and 2.4% (95% CI, 1.5-3.5%), over three times higher than the first period.
Similar to Klebsiella spp., Enterobacter spp. shows increasing resistance to imipenem and meropenem as well. Its rates are 0.4% (95% CI, 0.3-0.5%) and 0.2% (95% CI, 0.1-0.4%) respectively. When it come to the third period, the resistance rates markedly increased to 1.4% (95% CI, 1.1-1.7%) and 1.2% (95% CI, 0.8-1.7%).
E. coli exhibits the lowest carbapenems resistance rates among all the Enterobacteriaceae genera, and no obvious increase is found among imipenem resistance rates calculated from three periods. The prevalence of meropenem resistance shows escalating trend and it has risen to 0.5% (95% CI, 0.2-0.9%) in the third period, compared with the rates in previous two periods (0.1%).
Discussion
Enterobacteriaceae are inhabitants of the intestinal flora and important pathogens in both nosocomial and community settings. As illustrated in this study, CRE exhibits rapid and extensive dissemination across Asian area. This may be caused by the factors listed below. Firstly, in terms of Enterobacteriaceae, they spread easily between humans by hand carriage as well as contaminated food and water; they also have a propensity to acquire genetic material through horizontal gene transfer, mediated mostly by plasmids and transposons (60,61). Secondly, among the main mechanisms causing carbapenem resistance in Enterobacteriaceae, the acquisition of specific genes encoding carbapenemases plays the most important role (62). Lots of research papers show that genes encoding carbapenemases are mostly plasmid-located and associated with various mobile genetic structures, such as transposons or integrons (63). Such a characteristic certainly accelerates inter-/intra-species dissemination of carbapenemase genes. Thirdly, the high prevalence of ESBLs and the limited therapeutic options to MDR infections increase the consumption of carbapenems. CRE are the successful products under the drug selective pressure. Other factors, such as the international travel and medical tourism, long term hospitalization and frequent use of invasive medical devices, have also fueled the rapid rise in carbapenems resistance (64).
According to our research, seven countries, including Vietnam, Thailand, South Korea, Japan, China, India and Turkey, provide national or multi-centers surveillance data. Studies performed in other countries are mostly conducted in single institutions or a small number of tertiary care hospitals. As we all know, most countries in Asia are developing countries with poor medical and health conditions, and the prevalence of CRE in such area are not optimistic. Therefore, there’s urgent need to establish more and better surveillance in Asian countries to obtain a clearer and more accurate pictures of this situation.
The present study demonstrates the increasing prevalence of CRE from a whole view of Asia, where data from each country (or regions) also shows consistent trends. In mainland China, according to the report of Mohanrin, the impenem resistance of E. coli and K. pneumonia in 2004-2005 is 0.0% and 0.7% (65), while the rate increases to 0.5% and 2.7% in 2010 (66). In Korea, the carbapenems resistance also follows such tendency. As the result of the KONSAR (67), E. coli and K. pneumonia is completely sensitive to impenem in 2000, while in 2009, the resistance rates come to 0.1% and 0.5% respectively (68).
Besides the common trend and resistance level, geographic variation also exhibits in some regions. A report in the UAE shows the rates of resistance to imipenem in E. coli and Klebsiella spp. are 35.7% and 29.8% respectively (1), much higher than the average rates. This phenomenon alarms us that it is important to assess susceptibility patterns by a specific country or region because they may provide more accurate data for treatment and control of CRE infections in local area.
Klebsiella spp. accounts for the largest proportion of carbapenem resistance in Enterobacteriaceae, namely 39.3%. This outcome is not surprising with the fact Klebsiella spp. is not only the leading cause of nosocomial infections but also a notorious “collector” of multidrug resistance plasmids. As the most important carbapenemases, KPC enzymes disseminate mainly among Klebsiella spp., and result in decreased susceptibility to carbapenems (69). Antibiotic treatment to carbapenem resistant Klebsiella spp. is limited to a few choices, typically including colistin, tigecycline, and one or more aminoglycoside. Further research is needed to investigate the best therapy to these patients.
Among all the Enterobacteriaceae species collected, Serratia spp. exhibits the highest carbapenems resistance rates. It is an opportunistic pathogen with ability to survive and grow under extreme conditions (70). During the past decades, Serratia spp. has played a more and more important role in the nosocomial infections, and has already become a great threat to patients’ health with mortality rates of approximately 40-50% in S. marcescens bacteremia (71,72). In China, the prevalence of carbapenem resistance in S. marcescens has risen to 5.6% in 2008, obviously higher than other genus (73). The carbapenems resistance in Serratia spp. was found highly related to chromosomally encoded β-lactamases, SME (74). Three point mutant variants of SME (SME-1, SME-2 and SME-3) have been sporadically isolated throughout the world (75,76). These enzymes significantly hydrolyse penicillins, cephalosporins and carbapenems and are inhibited by clavulanic acid. All these facts above alarm us that more attention should be focused on Serratia spp., a potential reservoir for carbapenems resistant Enterobacteriaceae.
We have recognized several limitations associated with this systematic review. Firstly, systematic reports in developing counties are rare while the hygiene conditions in those countries are often worrisome. So the real situation of CRE in Asia may be underestimated. Secondly, biased sampling may not be totally excluded. For instance, some studies have been conducted in a single institution or a small number of tertiary care hospitals covering limited times. Thirdly, the method of antimicrobial susceptibility test and interpretation of the result also influence the resistant rates. At last, the change of CLSI breakpoints in the year of 2010 may increase the carbapenems resistant rates in Enterobacteriaceae. Despite these limitations, this study represented a current assessment of the prevalence of carbapenems-resistant Enterobacteriaceae across the Asian area.
The main preventive efforts for containing the emergence and spread of carbapenem-resistant Enterobacteriaceae could be directed at antimicrobial stewardship and infection control. A case-case control investigation has found that antimicrobial exposure is the strongest independent predictor for CRE isolation and concluded that antimicrobial stewardship plays a pivotal role in preventing CRE isolation (77). Therefore, optimizing and limiting unnecessary antimicrobial use are great weapons for us to fight against the emergence of CRE.
Interventions such as hand hygiene, contact precautions, healthcare personnel education, minimizing device use and so on are recommended in the guidelines published in USA and Europe for interventions to control CRE transmission in health care facilities (78,79). What’s more, two important approaches emphasized in the guidelines are: to recognize CRE as epidemiologically important and to understand the prevalence in their region. As illustrated in this study, it still needs lots of work to establish better surveillance programs in most Asian countries. So we advocate that surveillance programs would be set up Asia-wide and even worldwide. Only when we cooperate together to prevent the diffusion of CRE from every link, can we survive in this storm of CRE.
Acknowledgements
Funding: This research was funded by National Natural Science Foundation of China (No. 81000754), a grant from the Key Laboratory for Laboratory Medicine of Jiangsu Province of China (No. XK201114) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Disclosure: The authors declare no conflict of interest.
References
- Al-Dhaheri AS, Al-Niyadi MS, Al-Dhaheri AD, et al. Resistance patterns of bacterial isolates to antimicrobials from 3 hospitals in the United Arab Emirates. Saudi Med J 2009;30:618-23. [PubMed]
- Khorasani G, Salehifar E, Eslami G. Profile of microorganisms and antimicrobial resistance at a tertiary care referral burn centre in Iran: emergence of Citrobacter freundii as a common microorganism. Burns 2008;34:947-52. [PubMed]
- Perez F, Van Duin D. Carbapenem-resistant Enterobacteriaceae: a menace to our most vulnerable patients. Cleve Clin J Med 2013;80:225-33. [PubMed]
- Daikos GL, Petrikkos P, Psichogiou M, et al. Prospective observational study of the impact of VIM-1 metallo-beta-lactamase on the outcome of patients with Klebsiella pneumoniae bloodstream infections. Antimicrob Agents Chemother 2009;53:1868-73. [PubMed]
- Ben-David D, Kordevani R, Keller N, et al. Outcome of carbapenem resistant Klebsiella pneumoniae bloodstream infections. Clin Microbiol Infect 2012;18:54-60. [PubMed]
- Polwichai P, Dejsirilert S, Panpetch S, et al. Antimicrobial resistance of Escherichia coli isolated from urine in Thailand from 2000 to 2005. J Med Assoc Thai 2009;92 Suppl 4:S59-67. [PubMed]
- Lee DS, Choe HS, Lee SJ, et al. Antimicrobial susceptibility pattern and epidemiology of female urinary tract infections in South Korea, 2010-2011. Antimicrob Agents Chemother 2013;57:5384-93. [PubMed]
- Shakya P, Barrett P, Diwan V, et al. Antibiotic resistance among Escherichia coli isolates from stool samples of children aged 3 to 14 years from Ujjain, India. BMC Infect Dis 2013;13:477-82. [PubMed]
- Navon-Venezia S, Chmelnitsky I, Leavitt A, et al. Plasmid-mediated imipenem-hydrolyzing enzyme KPC-2 among multiple carbapenem-resistant Escherichia coli clones in Israel. Antimicrob Agents Chemother 2006;50:3098-101. [PubMed]
- McGann P, Hang J, Clifford RJ, et al. Complete sequence of a novel 178-kilobase plasmid carrying bla(NDM-1) in a Providencia stuartii strain isolated in Afghanistan. Antimicrob Agents Chemother 2012;56:1673-9. [PubMed]
- Poirel L, Al Maskari Z, Al Rashdi F, et al. NDM-1-producing Klebsiella pneumoniae isolated in the Sultanate of Oman. J Antimicrob Chemother 2011;66:304-6. [PubMed]
- Cho SH, Shin HH, Choi YH, et al. Enteric bacteria isolated from acute diarrheal patients in the Republic of Korea between the year 2004 and 2006. J Microbiol 2008;46:325-30. [PubMed]
- Islam MA, Talukdar PK, Hoque A, et al. Emergence of multidrug-resistant NDM-1-producing Gram-negative bacteria in Bangladesh. Eur J Clin Microbiol Infect Dis 2012;31:2593-600. [PubMed]
- Vlieghe ER, Phe T, De Smet B, et al. Bloodstream infection among adults in Phnom Penh, Cambodia: key pathogens and resistance patterns. PLoS One 2013;8:e59775. [PubMed]
- Biedenbach DJ, Bouchillon SK, Hoban DJ, et al. Antimicrobial susceptibility and extended-spectrum beta-lactamase rates in aerobic gram-negative bacteria causing intra-abdominal infections in Vietnam: report from the Study for Monitoring Antimicrobial Resistance Trends (SMART 2009-2011). Diagn Microbiol Infect Dis 2014;79:463-7. [PubMed]
- Tan TY, Hsu LY, Koh TH, et al. Antibiotic resistance in gram-negative bacilli: a Singapore perspective. Ann Acad Med Singapore 2008;37:819-25. [PubMed]
- Radji M, Fauziah S, Aribinuko N. Antibiotic sensitivity pattern of bacterial pathogens in the intensive care unit of Fatmawati Hospital, Indonesia. Asian Pac J Trop Biomed 2011;1:39-42. [PubMed]
- Cao V, Lambert T, Nhu DQ, et al. Distribution of extended-spectrum beta-lactamases in clinical isolates of Enterobacteriaceae in Vietnam. Antimicrob Agents Chemother 2002;46:3739-43. [PubMed]
- Litzow JM, Gill CJ, Mantaring JB, et al. High frequency of multidrug-resistant gram-negative rods in 2 neonatal intensive care units in the Philippines. Infect Control Hosp Epidemiol 2009;30:543-9. [PubMed]
- Salvador VB, Lozada MC, Consunji RJ. Microbiology and antibiotic susceptibility of organisms in bile cultures from patients with and without cholangitis at an Asian academic medical center. Surg Infect (Larchmt) 2011;12:105-11. [PubMed]
- Raja NS. Microbiology of diabetic foot infections in a teaching hospital in Malaysia: a retrospective study of 194 cases. J Microbiol Immunol Infect 2007;40:39-44. [PubMed]
- Huh K, Kim J, Cho SY, et al. Continuous increase of the antimicrobial resistance among gram-negative pathogens causing bacteremia: a nationwide surveillance study by the Korean Network for Study on Infectious Diseases (KONSID). Diagn Microbiol Infect Dis 2013;76:477-82. [PubMed]
- Lee K, Park KH, Jeong SH, et al. Further increase of vancomycin-resistant Enterococcus faecium, amikacin- and fluoroquinolone-resistant Klebsiella pneumoniae, and imipenem-resistant Acinetobacter spp. in Korea: 2003 KONSAR surveillance. Yonsei Med J 2006;47:43-54. [PubMed]
- Lee K, Lim CH, Cho JH, et al. High prevalence of ceftazidime-resistant Klebsiella pneumoniae and increase of imipenem-resistant Pseudomonas aeruginosa and Acinetobacter spp. in Korea: a KONSAR program in 2004. Yonsei Med J 2006;47:634-45. [PubMed]
- Lee K, Lee MA, Lee CH, et al. Increase of ceftazidime- and fluoroquinolone-resistant Klebsiella pneumoniae and imipenem-resistant Acinetobacter spp. in Korea: analysis of KONSAR study data from 2005 and 2007. Yonsei Med J 2010;51:901-11. [PubMed]
- Lee K, Kim YA, Park YJ, et al. Increasing prevalence of vancomycin-resistant enterococci, and cefoxitin-, imipenem- and fluoroquinolone-resistant gram-negative bacilli: a KONSAR study in 2002. Yonsei Med J 2004;45:598-608. [PubMed]
- Lee K, Jang SJ, Lee HJ, et al. Increasing prevalence of vancomycin-resistant Enterococcus faecium, expanded-spectrum cephalosporin-resistant Klebsiella pneumoniae, and imipenem-resistant Pseudomonas aeruginosa in Korea: KONSAR study in 2001. J Korean Med Sci 2004;19:8-14. [PubMed]
- Ohnishi M, Sawada T, Hirose K, et al. Antimicrobial susceptibilities and bacteriological characteristics of bovine Pseudomonas aeruginosa and Serratia marcescens isolates from mastitis. Vet Microbiol 2011;154:202-7. [PubMed]
- Ishii Y, Alba J, Kimura S, et al. Evaluation of antimicrobial activity of beta-lactam antibiotics by Etest against clinical isolates from 100 medical centers in Japan (2004). Diagn Microbiol Infect Dis 2006;55:143-8. [PubMed]
- Ishii Y, Alba J, Kimura S, et al. Evaluation of antimicrobial activity of beta-lactam antibiotics using Etest against clinical isolates from 60 medical centres in Japan. Int J Antimicrob Agents 2005;25:296-301. [PubMed]
- Ishii Y, Ueda C, Kouyama Y, et al. Evaluation of antimicrobial susceptibility for beta-lactams against clinical isolates from 51 medical centers in Japan (2008). Diagn Microbiol Infect Dis 2011;69:443-8. [PubMed]
- Ishii Y, Tateda K, Yamaguchi K. Evaluation of antimicrobial susceptibility for beta-lactams using the Etest method against clinical isolates from 100 medical centers in Japan (2006). Diagn Microbiol Infect Dis 2008;60:177-83. [PubMed]
- Takesue Y, Watanabe A, Hanaki H, et al. Nationwide surveillance of antimicrobial susceptibility patterns of pathogens isolated from surgical site infections (SSI) in Japan. J Infect Chemother 2012;18:816-26. [PubMed]
- Cheng NC, Hsueh PR, Liu YC, et al. In vitro activities of tigecycline, ertapenem, isepamicin, and other antimicrobial agents against clinically isolated organisms in Taiwan. Microb Drug Resist 2005;11:330-41. [PubMed]
- Jean SS, Hsueh PR, Lee WS, et al. Nationwide surveillance of antimicrobial resistance among Enterobacteriaceae in intensive care units in Taiwan. Eur J Clin Microbiol Infect Dis 2009;28:215-20. [PubMed]
- Xiao YH, Wang J, Zhao CY, et al. Mohnarin bacterial resistance surveillance 2006-2007. Chin J Nosocomiol 2008;18:1051-6.
- Wang Q, Zhao CJ, Wang H, et al. Antimicrobial resistance of Gram-negative bacilli isolated from 13 teaching hospitals across China. Zhonghua Yi Xue Za Zhi 2013;93:1388-96. [PubMed]
- Chen HB, Zhao ChQ, Wang H, et al. Ananalysis ofresistance ofnosocomial infection pathogens isolated from 13 teaching hospitals in 2011. Zhonghua Nei Ke Za Zhi 2013;52:203-12. [PubMed]
- Ling TK, Xiong J, Yu Y, et al. Multicenter antimicrobial susceptibility survey of gram-negative bacteria isolated from patients with community-acquired infections in the People's Republic of China. Antimicrob Agents Chemother 2006;50:374-8. [PubMed]
- Chen BH, Zhang XJ, Zhao Y, et al. National Antimicrobial Resistant Investigation Net Mohnarin) 2009 annual report: Antimicrobial resistance surveillance in intensive care units. Chin J Clin Pharmacol 2011;27:483-9.
- Gao L, Li Y. Mohnarin 2009 report: Bacterial drug resistant surveillance of non-ICU inpatients. Chin J Clin Pharmacol 2011;27:373-9.
- Chu YZ, Tian SF, Chen BY, et al. Pharmacokinetic-pharmacodynamic profiling of four antimicrobials against gram-negative bacteria collected from Shenyang, China. BMC Infect Dis 2010;10:171-5. [PubMed]
- Jones RN, Rhomberg PR, Varnam DJ, et al. A comparison of the antimicrobial activity of meropenem and selected broad-spectrum antimicrobials tested against multi-drug resistant Gram-negative bacilli including bacteraemic Salmonella spp.: initial studies for the MYSTIC programme in India. Int J Antimicrob Agents 2002;20:426-31. [PubMed]
- Kothari A, Sagar V. Antibiotic resistance in pathogens causing community-acquired urinary tract infections in India: a multicenter study. J Infect Dev Ctries 2008;2:354-8. [PubMed]
- Pathak A, Marothi Y, Kekre V, et al. High prevalence of extended-spectrum β-lactamase-producing pathogens: results of a surveillance study in two hospitals in Ujjain, India. Infect Drug Resist 2012;5:65-73. [PubMed]
- Rizvi MF, Hasan Y, Memon AR, et al. Pattern of nosocomial infection in two intensive care units of a tertiary care hospital in Karachi. J Coll Physicians Surg Pak 2007;17:136-9. [PubMed]
- Ali SA, Tahir SM, Memon AS, et al. Pattern of pathogens and their sensitivity isolated from superficial surgical site infections in a tertiary care hospital. J Ayub Med Coll Abbottabad 2009;21:80-2. [PubMed]
- Shree N, Arora BS, Mohil RS, et al. Bacterial profile and patterns of antimicrobial drug resistance in intra-abdominal infections: current experience in a teaching hospital. Indian J Pathol Microbiol 2013;56:388-92. [PubMed]
- Khorvash F, Mostafavizadeh K, Mobasherizadeh S, et al. Antimicrobial susceptibility pattern of microorganisms involved in the pathogenesis of surgical site infection (SSI); A 1 year of surveillance. Pak J Biol Sci 2008;11:1940-4. [PubMed]
- Jamal WY, Al Hashem G, Khodakhast F, et al. Comparative in vitro activity of tigecycline and nine other antibiotics against gram-negative bacterial isolates, including ESBL-producing strains. J Chemother 2009;21:261-6. [PubMed]
- Shahcheraghi F, Moezi H, Feizabadi MM. Distribution of TEM and SHV beta-lactamase genes among Klebsiella pneumoniae strains isolated from patients in Tehran. Med Sci Monit 2007;13:BR247-50. [PubMed]
- Mehrgan H, Rahbar M, Arab-Halvaii Z. High prevalence of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in a tertiary care hospital in Tehran, Iran. J Infect Dev Ctries 2010;4:132-8. [PubMed]
- Al-Tawfiq JA. Increasing antibiotic resistance among isolates of Escherichia coli recovered from inpatients and outpatients in a Saudi Arabian hospital. Infect Control Hosp Epidemiol 2006;27:748-53. [PubMed]
- Kuzucu C, Yetkin F, Görgeç S, et al. Investigation of the susceptibilities of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella spp. strains to ertapenem and other carbapenems. Mikrobiyol Bul 2011;45:28-35. [PubMed]
- Al-Zarouni M, Senok A, Rashid F, et al. Prevalence and antimicrobial susceptibility pattern of extended-spectrum beta-lactamase-producing Enterobacteriaceae in the United Arab Emirates. Med Princ Pract 2008;17:32-6. [PubMed]
- Mehrgan H, Rahbar M. Prevalence of extended-spectrum beta-lactamase-producing Escherichia coli in a tertiary care hospital in Tehran, Iran. Int J Antimicrob Agents 2008;31:147-51. [PubMed]
- Ahmad S, Al-Juaid NF, Alenzi FQ, et al. Prevalence, antibiotic susceptibility pattern and production of extended-spectrum beta-lactamases amongst clinical isolates of Klebsiella pneumoniae at Armed Forces Hospital in Saudi Arabia. J Coll Physicians Surg Pak 2009;19:264-5. [PubMed]
- Bazzaz BS, Naderinasab M, Mohamadpoor AH, et al. The prevalence of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae among clinical isolates from a general hospital in Iran. Acta Microbiol Immunol Hung 2009;56:89-99. [PubMed]
- Emamghorashi F, Farshad S, Kalani M, et al. The prevalence of O serogroups of Escherichia coli strains causing acute urinary tract infection in children in Iran. Saudi J Kidney Dis Transpl 2011;22:597-601. [PubMed]
- Partridge SR. Analysis of antibiotic resistance regions in Gram-negative bacteria. FEMS Microbiol. Rev 2011;35:820-55. [PubMed]
- Stokes HW, Gillings MR. Gene flow, mobile genetic elements and the recruitment of antibiotic resistance genes into Gram-negative pathogens. FEMS Microbiol Rev 2011;35:790-819. [PubMed]
- Nordmann P, Dortet L, Poirel L. Carbapenem resistance in Enterobacteriaceae: here is the storm! Trends Mol Med 2012;18:263-72. [PubMed]
- Cuzon G, Naas T, Nordmann P. Functional characterization of Tn4401, a Tn3-based transposon involved in blaKPC gene mobilization. Antimicrob Agents Chemother 2011;55:5370-73. [PubMed]
- Castanheira M, Deshpande LM, Mathai D, et al. Early dissemination of NDM-1- and OXA-181-producing Enterobacteriaceae in Indian hospitals: report from the SENTRY Antimicrobial Surveillance Program, 2006-2007. Antimicrob Agents Chemother 2011;55:1274-8. [PubMed]
- Xiao YH, Wang J, Li Y. Bacterial resist ance surveillance in China: a report from Mohnarin 2004-2005. Eur J Clin Microbiol Infect Dis 2008;27:697-708. [PubMed]
- Xiao YH, Shen P, Wei ZQ. Mohnrin report of 2010: surveillance of bacterial resistance in China. Chin J Nosocomiol 2011;21:4896-902.
- Lee KW, Kim MY, Kang SH, et al. Korean nationwide surveillance of antimicrobial resistance in 2000 with special reference to vancomycin resistance in enterococci, and expanded-spectrum cephalosporin and imipenem resistance in gram-negative bacilli. Yonsei Med J 2003;44:571-8. [PubMed]
- Lee K, Kim MN, Kim JS, et al. Further increases in carbapenem-, amikacin-, and fluoroquinolone-resistant isolates of Acinetobacter spp. and P. aeruginosa in Korea: KONSAR study 2009. Yonsei Med J 2011;52:793-802. [PubMed]
- Tzouvelekis LS, Markogiannakis A, Psichogiou M, et al. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 2012;25:682-707. [PubMed]
- Šiširak M, Hukić M. An outbreak of multidrug-resistant Serratia marcescens: the importance of continuous monitoring of nosocomial infections. Acta Med Acad 2013;42:25-31. [PubMed]
- Yu WL, Lin CW, Wang DY. Serratia marcescens bacteremia: clinical features and antimicrobial susceptibilities of the iso-lates. J Microbiol Immunol Infect 1998;31:171-9. [PubMed]
- Shih HI, Lee HC, Lee NY, et al. Serratia marcescens bacteremia at a medical center insouthern Taiwan: high prevalence of cefotaxime resistance. J Microbiol Immunol Infect 2005;38:350-7. [PubMed]
- Xiao YH, Wang J, Zhu Y, et al. Mohnarin of 2008: Surveillance Results of National Bacterial Drug Resistance. Chinese Journal of Nosocomiology 2010;20:2377-83.
- Naas T, Vandel L, Sougakoff W, et al. Cloning and sequence analysis of the gene for a carbapenem-hydrolyzing class Aβ-lactamase, Sme-1, from Serratia marcescensS6. Antimicrob Agents Chemother 1994;38:1262-70. [PubMed]
- Queenan AM, Torres-Viera C, Gold HS, et al. SME-type carbapenem-hydrolyzing class A β-lactamases from geographically diverse Serratia marcescens strains. Antimicrob Agents Chemother 2000;44:3035-9. [PubMed]
- Poirel L, Wenger A, Bille J, et al. SME-2-producing Serratia marcescensisolate from Switzerland. Antimicrob Agents Chemother 2007;51:2282-3. [PubMed]
- Marchaim D, Chopra T, Bhargava A, et al. Recent exposure to antimicrobials and carbapenem-resistant Enterobacteriaceae: the role of antimicrobial stewardship. Infect Control Hosp Epidemiol 2012;33:817-30. [PubMed]
- Centers for Disease Control and Prevention (CDC). Guidance for control of infections with carbapenem-resistant or carbapenemase-producing Enterobacteriaceae in acute care facilities. MMWR Morb Mortal Wkly Rep 2009;58:256-60. [PubMed]
- Kallen A, Guh A. United States Centers for Disease Control and Prevention issue updated guidance for tackling carbapenem-resistant Enterobacteriaceae. Euro Surveill 2012;17:17-26. [PubMed]