Long-term continuous-flow left ventricular assist devices (LVAD) as bridge to heart transplantation
Introduction
Heart transplantation (HTx) is the treatment of choice for end-stage heart failure and the number of reported HTx now appears to be slowly increasing, particularly in North America (1). However the shortage of heart’s donors still represents a major issue. So long-term mechanical circulatory support (MCS) has been proposed as an alternative treatment option to assist patients scheduled on HTx waiting list bridging them for a variable time period to cardiac transplantation—the so-called bridge-to-transplantation (BTT) strategy.
Long-term MCS can be classified according to the type of assistance in left ventricular assist device (LVAD), biventricular assist device (BiVAD) and total artificial heart (TAH). Nowadays approximately 90% of patients being considered for MCS receive an LVAD (2). In fact, LVAD experienced several improvements in the last decade and the predominance of continuous-flow over pulsatile-flow technology has been evident since 2008 [>95% of all patients receiving primary MCS implants (2)]. So the aim of the present report is to give an overview of continuous-flow LVAD utilization in the specific setting of the BTT strategy taking into consideration the most representative articles of the scientific literature.
Continuous-flow axial LVAD
The “first generation” pulsatile-flow LVAD were engineered with an internal reservoir chamber and inflow and outflow valves that permit cyclic filling and emptying of the device, mimicking the physiologic systole and diastole of the native heart (3). Pulsatile-flow LVAD have been progressively replaced by continuous-flow devices. The “second generation” continuous-flow LVAD have an axial blood flow path with an internal rotor within the blood flow that is suspended by contact bearings (4).
HeartMate II
The HeartMate II (Thoratec, Pleasanton, CA, USA) was first evaluated in a European study that ended early due to poor outcomes related to device design issues [thrombus formation at the inlet and outlet stators (5)]. On the basis of the problems encountered, the HeartMate II was redesigned and utilized for the first time as BTT in 2003 (6). Frazier and co-workers published in 2007 the first single-centre experience with this device [43 patients, 60.4% as BTT (7)]. The initial experience was favourable and they did not encounter any pump failures or operational problems.
In the same year Miller and co-workers published the pivotal, multicentre clinical trial on the HeartMate II utilization as BTT in a population of 133 patients (8). Fifty-six (42.1%) patients were successfully bridged to HTx after a median support time of 97 days while 25 (19%) died while on support. During a period of support of at least 6 months, patients experienced a substantial improvement of the functional status and quality of life. After this pivotal trial, the investigators reported on the results of the first 281 patients entered into a continued-access protocol approved by the Food and Drug Administration (FDA) and with at least 18 months of follow-up (9). The results of this study have validated the efficacy and safety profile of the HeartMate II for patients awaiting HTx. At 18 months after LVAD implantation the vast majority of patients (79%) underwent HTx, had cardiac recovery or remained alive with ongoing LVAD support. Among the patients remaining alive with ongoing LVAD support, the majority were actively listed for HTx, obtained satisfactory renal and hepatic function and functional status and were free from significant neurological complications.
In April 2008 HeartMate II device became in the United States the first continuous-flow LVAD approved as a BTT. Finally, Starling and colleagues published in 2011 the results of the post-approval study in comparison with other FDA-approved, pulsatile devices for BTT (10). The study evaluated the first 169 consecutive HeartMate II patients enrolled after the FDA approval in the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) database. The comparison group (n=169) encompassed the electric HeartMate XVE LVAD (n=135) and the pneumatic Thoratec Implantable Ventricular Assist Device (n=34). Overall survival at 1, 6 and 12 months was better in the HeartMate II group and a greater percentage of patients in the HeartMate II (90%, n=152) versus the comparison (80%, n=130) group reached the successful outcomes of survival to HTx, recovery of the heart or ongoing support at 6 months (P=0.018). So this study confirmed the initial results of the pivotal clinical trial also in a real world BTT population and showed a constant trend towards better short- and intermediate-term survival. The efficacy, reliability and utility of the HeartMate II in a real world BTT patient population was also reported by John et al. in a single-centre study over a 5-year time period (11). Tables 1 and 2 resume the most important findings and the most frequent adverse events of the HeartMate II utilization as BTT.
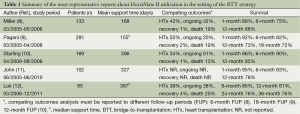
Full table
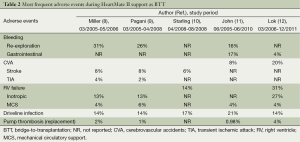
Full table
Jarvik 2000
The Jarvik 2000 (Jarvik Heart Inc., New York, NY, USA) is a miniaturized continuous-flow LVAD weighing only 90 gr and implanted in apical intraventricular position. The implant can be performed through a standard median sternotomy or a left thoracotomy; in the left thoracotomy approach the outflow graft is anastomosed to the descending thoracic aorta. The Jarvik 2000 displays the peculiar feature that the power cable may exit at the apex of the chest in the first intercostal space and passes then through the neck to a titanium pedestal screwed into the skull behind the mastoid process; this percutaneous pedestal, based on cochlear implant technology, transmits the cable to an external portable controller and battery (13).
The first encouraging results of this device bridging safely and satisfactorily patients to HTx date to 2000 and were performed at the Texas Heart Institute (14-16).
In an American and European multicentre study evaluating the mechanical reliability of the Jarvik 2000, 83 patients had the device implanted with an abdominal power supply as BTT (17). No mechanical failure of the pumps or any implantable components occurred in the study population over a mean period of support of 159 days.
The adverse events of this axial LVAD were well detailed in single-centre study enrolling 22 patients (18). Re-exploration for bleeding was the most frequent adverse event (40.9%) followed by stroke (22.7%). Right ventricular failure requiring MCS, gastrointestinal bleeding and driveline infection were encountered in only one patient (4.5%), respectively. There were no cases of pump thrombosis. In this experience the rate of acute postoperative bleeding, despite minor surgical trauma, was high and the authors stated that it could be explained partly by the high incidence of preoperative liver dysfunction among the patients.
Finally, the surgical team of the University of Maryland published in 2012 the second largest USA experience with the Jarvik 2000 (19). They implanted 35 devices in a study-period of 8 years and, as a comparison group, they selected 30 consecutive HeartMate II recipients. Compared with the Heartmate II population, Jarvik 2000 insertion was associated with significantly fewer intraoperative packed red blood cells transfusions but total operative time for on-pump insertion tended to be significantly longer in patients undergoing Jarvik 2000 implantation despite similar cardiopulmonary bypass times. However, overall survival did not differ by device. Moreover, intraoperative transfusions at the time of LVAD insertion and total intensive care unit stay after LVAD insertion were found by multiple regression to be significantly reduced for patients who had undergone previous sternotomy and received a Jarvik 2000 implant through a left thoracotomy when compared with patients requiring repeat sternotomy for implantation of either the Jarvik 2000 or the HeartMate II.
Continuous-flow centrifugal LVAD
The “3rd generation” continuous-flow LVAD are rotary devices with an impeller or rotor suspended in the blood flow path using a “noncontact” bearing design. In the majority of cases, this design utilizes a “centrifugal” blood flow path and incorporates either magnetic and/or hydrodynamic levitation of the internal impeller. The only exception is represented by the Incor (Berlin Heart GmbH, Berlin, Germany), which is a “3rd generation” rotary pump with an axial blood flow path and magnetic levitation of the internal rotor (3).
HeartWare
The HeartWare (HeartWare Inc., Framingham, MA, USA) has the unique feature of a small design size. It has a displacement volume of 45 mL and weighs 145 g with a flow capacity of up to 10 L/min. The device is small enough to take place within the pericardial cavity without the need for dissection and creation of a preperitoneal pocket (3).
The safety and efficacy of the HeartWare when used as a BTT were evaluated in a multicentre, prospective, nonrandomized, single-arm trial on a population of 50 patients (20). Twenty (40%) patients were successfully bridged to HTx after a median support time of 267 days while 9 (18%) died while on support. The causes of death were sepsis (n=3), multiple organ failure (n=3) and hemorrhagic stroke (n=3). The HeartWare provided an effective hemodynamic support, witnessed by the fact that renal and hepatic function were abnormal after the implant surgery but returned to normal ranges by 2 weeks and evidenced by the improvement in measures of quality of life and neurocognitive function.
Aaronson and co-workers conducted a multicentre, prospective, study comparing success and survival of the HeartWare (n=140) against a control group (n=499) drawn from the INTERMACS registry (21). The HeartWare yielded a 90.7% probability of success at 180 days, a result non-inferior to that of control patients receiving commercially available implanted LVAD. Moreover, infection, right heart failure, device replacement, stroke, kidney dysfunction, hemolysis and arrhythmia rates for the HeartWare were similar to those reported previously for the HeartMate II (9). After completion of enrollment in this pivotal trial, an additional 256 patients were enrolled to receive a HeartWare as BTT under a continued access protocol. Slaughter et al. reported in 2013 the analysis of combined data from the 140 BTT patients plus 192 patients from the continued access protocol (22). These data continued to support the findings from the HeartWare BTT pivotal trial regarding the safety and efficacy of the device in patients with end-stage heart failure requiring an LVAD as a BTT and adverse event rates compared favourably with historical rates of LVAD as BTT. Finally, the results of a prospective, post-market registry of patients receiving the HeartWare system at nine centres in Europe and Australia were published recently by Strueber and colleagues (23). Data from this study demonstrate continued high-level safety and performance of the HeartWare with survival rates comparing favourably to survival reported previously for both the HeartWare and other commercially available LVAD. The adverse event profile has exhibited improvement since the clinical trials (20-22,24), witnessing excellent outcomes when moving from clinical trial setting to commercial, real-world. Tables 3 and 4 resume the most important findings and the most frequent adverse events of the HeartWare utilization as BTT.
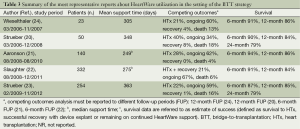
Full table
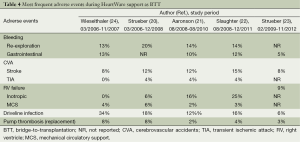
Full table
At the beginning of the experience, a series of pumps developed unexplained thrombi (20) and, so, HeartWare reviewed the anticoagulation therapy protocol in March 2011 (22). In fact, at that time, most patients with HeartWare thrombus had sub-therapeutic international normalized ratio (INR) values and were taking 81 mg aspirin or no acetylsalicylic acid. Hence, the principal investigators recommended in March 2011 that strict adherence to the protocol INR requirement of 2.0 to 3.0 be followed and that the acetylsalicylic acid dose be advanced to 325 mg. After the anticoagulation adjustment, the annualized rate of pump exchange for suspected thrombus dropped of 55%, the incidence of ischemic strokes resulting in any level of neurologic disability declined from 5.1% to 2.8% while the incidence of hemorrhagic strokes resulting in any level of neurologic disability was relatively unchanged (from 5.1% to 4.7%) (22). Moreover, since May 2011, the exterior surface of the inflow cannula was modified to include sintered titanium microspheres; the goal was to prevent tissue growth progression beyond the sintered area of the cannula. Concurrently with the introduction of the sintered pump, a coring tool was introduced that increased the coring diameter from 16 to 19 mm, which provided cleaner cuts along the edges of the myocardium into which the inflow cannula is inserted (25). The observed pump thrombus event rate did not differ significantly between the patients who received a sintered HeartWare and those who received the original non-sintered pump but it is possible that a longer observation period is required to better evaluate the effect of sintering on the occurrence of late pump thrombus events (25).
DuraHeart
The DuraHeart (Terumo Heart Inc, Ann Arbor, MI, USA) is the world’s first approved magnetically levitated continuous-flow centrifugal LVAD designed for long-term MCS. In this pump there is the elimination of all mechanical contacts inside the blood chamber.
In 2009 was published the first European multicentre study on DuraHeart to obtain the Conformité Européenne (CE) approval in patients candidates to HTx (26). The primary endpoint of the study was to evaluate the survival of the patient either to HTx or at 3 months of the device support. Secondary endpoints evaluated adverse events, device performance and overall patient status throughout the period of support. Sixty-eight patients were eligible; they were adults with end-stage left ventricular failure at imminent risk of dying and on maximal conventional therapy but unresponsive to medical treatment. The patients were divided in two groups: 33 patients were enrolled in CE-mark study and 35 patients were enrolled in post-market study. In the CE-mark trial patients, 82% reached the primary endpoint of HTx or survival at 13 weeks endpoint. Two patients who were replaced with a second DuraHeart device and kept alive were included as survivors in the survival analysis. Three additional deaths occurred at 239, 549 and 550 days. The mean support duration was 338±311 days. Fourteen patients (42%) were transplanted with a mean time to HTx of 185±148 days. Nine patients (27%) still remained on device support with a mean duration of 744±216 days with 13 patients supported >1 year. Of these 13 patients, 4 were supported >2 years and 1 was supported >3 years. Kaplan-Meir survival estimates for the CE-mark study were 81% at 3 months, 77% at 6 months, 72% at 1 year and 57% at 2 years. Seven deaths occurred during device support, six before the primary endpoint and one after the primary endpoint. One ischemic and three hemorrhagic cerebrovascular accidents were determined to be the cause of death in 4 patients (57%) and the majority of deaths (6 patients, 85%) occurred in the initial 11 patients enrolled in the study. The pump and inflow/outflow conduits from the patients who died of cerebrovascular accidents were thoroughly analysed for thrombus formation, signs of infection or any abnormality. There was any evidence of pump thrombosis or infection in the blood path. Due to a high incidence of fatal intra-cerebral bleeding and other bleeding complications observed in the initial 11 patients, the anticoagulation and anti-platelet regimen was reviewed.
Recently the results of a prospective multicentre single-arm observational study were published: it is the US SUSTAIN trial (27). The cohort of the patients is very similar to the European multicentre study. The trial was closed before conclusion as a result of slow recruitment related to the size of the pump, difficulty in surgical implantation and provider preference as newer, smaller continuous flow devices gained wider acceptance. A total of 63 patients were enrolled in the study and implanted with the DuraHeart in 23 centres. The mean age was 54.8 years and 16% were females. The median duration of support was 267 days with a total support time of 46 patient-years. Follow-up to 180 days was complete on all patients. Of the 63 patients implanted, 16 (25%) underwent HTx and 30 (48%) were listed for HTx at the 6-month primary end point, resulting in a success rate of 73%. Of the 17 patients not reaching the primary end point, 9 (14%) died, 4 (6%) underwent device exchange and 4 (6%) were not listed for HTx at 180 days. The major adverse events in this trial were similar to those of the precedent study. Moreover the Investigators found 10% of cable wire fracture causing loss of magnetic levitation during the study period. Five wires broke near the connection to the pump and the sixth broke near an intermediate connector. In all cases the patients were hemodynamically stable as the device entered into the built-in backup hydrodynamic mode. Five patients underwent successful device exchange despite continued support in hydrodynamic mode owing to safety concerns after the loss of magnetic levitation; one patient underwent HTx.
Although the DuraHeart is no longer manufactured for use in the United States, the two studies shows the safety and performance of the DuraHeart. The results demonstrated that the DuraHeart was safe and is intended for MCS for patients at imminent risk of death due to end-stage left ventricular failure and eligible to HTx.
Discussion
Historical background
MCS emerged as an accepted and valuable treatment option for patients with advanced heart failure in the last two decades (28). Historically patients being considered for LVAD as BTT therapy have undergone the implantation of a “first generation” pulsatile-flow device. Nonetheless their efficacy, the utilization of these devices was associated with significant morbidity and mortality, which could be related to several factors such as a large pump size (needing for a large body habitus of the patient and an extensive surgical dissection), a large diameter of the percutaneous lead and a limited long-term durability and reliability. Bleeding and infection represented leading adverse events and, in particular, a major limitation of pulsatile-flow LVAD has been the high incidence (up to 65%) of reoperation for device replacement as a consequence of device infection, thrombosis or malfunction (29,30).
Evolution of LVAD technology and clinical implications
The introduction of the continuous-flow technology in the setting of LVAD represented a true innovation. First of all, these devices are smaller than previous pulsatile ones. The reduction of the size of the pump allowed their utilization in underserved population such as women and adolescents patients (7,31,32). Moreover smaller pumps do not need a large pre-peritoneal abdominal pocket, thus limiting the surgical dissection and reducing the potential risk of bleeding and infection (31). The reduction in size involved also the percutaneous driveline and determined less noise from the device and a greater comfort for the patients. Finally, another relevant advantage of continuous-flow devices is represented by the greater long-term durability and reliability. This is explicated by the simplified technology encompassing only a single moving part (i.e., the internal rotor). Device replacement is an infrequent complication and, when necessary, the main indications are represented by device thrombosis or infection, and mechanical failure is rarely encountered nowadays (8-11,17, 20).
In addition to the abovementioned advantages that are shared by the various second and third-generation continuous-flow LVAD, it is important to remember that there are specific characteristics that are instead peculiar to a given LVAD. The Jarvik 2000 can be implanted through a left thoracotomy approach and may facilitate the subsequent HTx by avoiding an additional sternotomy (19). Moreover, it avoids the need for redo sternotomy at implantation in those patients who have had such surgery. Its intraventricular position without inflow cannula, on the one hand, does not require any abdominal pump pocket reducing the risk of infection and bleeding and, on the other hand, could lower the risk of thromboembolism as a consequence of a reduced blood contact to foreign surfaces (13). The skull-mounted postauricular percutaneous pedestal is similar to that used for artificial ear implants, proved reliable and reduced the risk of driveline infection (33). Also the HeartWare implantation can be performed by a minimally invasive approach via a left thoracotomy (34) and this device is engineered with an integrated inflow cannula allowing for a complete intrapericardial placement (20).
Continuous-flow LVAD provide an effective hemodynamic support as hepatic and renal function test (9,12,20,24,35), functional status (8,9,21,22,36) and quality of life (8-10,20-22,36) improve significantly during long-term support. Moreover, the technical advances of the continuous-flow technology associated to improvements gained over time in patient selection, timing of LVAD implantation and postoperative patient management led to a reduction of adverse events. The adverse event rates of bleeding requiring surgical re-exploration, percutaneous driveline infection, cerebrovascular accidents and right heart failure requiring a right ventricular assist device were significantly less in continuous-flow LVAD compared with those observed during clinical evaluation of a pulsatile pump (9,28). Taken together these data could explain the amelioration of the survival observed since the first clinical trials of continuous-flow LVAD, highlighted also by longer mean support time and mean support time to HTx (8-12,20-24).
Continuous-flow LVAD and cardiac transplantation
The recently updated European Society of Cardiology guidelines for the treatment of acute and chronic heart failure added the use of LVAD as a Class I/B recommendation in patients deteriorating on medical therapy while waiting for HTx (37). Indeed, the use of LVAD to bridge patients to HTx continues to increase and has reached approximately 30% in the current era (1).
Nonetheless clinical results with continuous-flow LVAD have shown a constant improvement over time in terms of better survival, reduced adverse events and proven device durability and reliability, there have been conflicting reports on the impact of LVAD on post-transplant survival and MCS is nowadays recognized as a risk factor of early and late mortality after HTx (1). However, several studies support and validate the use of continuous-flow LVAD as BTT (38-41). In particular, continuous-flow LVAD provide post-transplant survival rates comparable to that of conventional HTx (38-41) and these acceptable post-transplant survival data are closely linked to the good outcomes during LVAD support as BTT.
Furthermore, in the setting of the BTT strategy the beneficial effects of continuous-flow LVAD seen in the post-transplant period are undeniable also before HTx, i.e., the time spent on the waiting list. Taghavi and colleagues analysed the waiting times and organ allocation in three different groups of patients: (I) patients supported with a continuous-flow LVAD; (II) patients supported with a pulsatile-flow LVAD; (III) patients without any type of MCS (42). Patients in the continuous-flow LVAD group had the longest total waitlist time compared with the pulsatile-flow LVAD and non-LVAD groups and experienced enhanced waitlist survival compared with the other groups. The clinical outcome of patients supported with a continuous-flow LVAD during the time spent on the waiting list coupled with their remarkable durability could allow for improved donor selection as opposed to the pulsatile pump era, in which decreasing durability beyond the 1-year mark increased the urgency for HTx and a subsequent potential for suboptimal donor selection (38).
Finally, LVAD allow a better management of those patients who are initially considered ineligible to HTx as a consequence of different contraindications such as, for example, pulmonary hypertension. Several studies have confirmed that pulmonary hypertension is a risk factor for early and late morbidity and mortality after HTx (43,44). Owing to the initial experience with pulsatile-flow LVAD (45-47), continuous-flow devices proved effective in significantly lowering pulmonary artery pressures, pulmonary vascular resistance and transpulmonary gradient during the period of support (48-52). The improvement of pulmonary hemodynamic was seen even in medically unresponsive or “fixed” pulmonary hypertension and maintained also after HTx (48-52). So LVAD allow patients with pulmonary hypertension to become eligible to HTx. In a current era of worldwide limited availability of heart’s donors, this bridge to candidacy strategy with proper patients’ evaluation and selection before HTx is crucial and of outmost importance.
Expanding the horizons of MCS as BTT: continuous-flow BIVAD
Biventricular heart failure refractory to maximal medical therapy and suitable to MCS could be managed with either a pulsatile BIVAD or a TAH. Patients with biventricular heart failure requiring MCS are more critically ill preoperatively, experience a significantly greater rate of adverse events during support and have a more adverse outcome overall than patients who receive an isolated LVAD (53,54). In order to transfer the advantages of the continuous-flow technology to biventricular heart failure patients, several reports were published about the possibility to use different continuous-flow LVAD for biventricular support (55-59). Moreover, Krabatsch et al. published the largest single-centre experience about HeartWare utilization as a BIVAD in 13 BTT patients (60). Eight (61.5%) patients could be discharged home after they recovered from the operation and in two patients, after 370 and 60 days of BIVAD support respectively, both right ventricular function and right ventricle end-diastolic diameter normalized, which resulted in frequent suction events between the inflow cannula and the opposite interventricular septum. Subsequently, thrombosis of the right pump occurred in both patients. The pumps were stopped without any hemodynamic consequences and the two patients remained on LVAD support only. This implantable continuous-flow BIVAD gives patients a greater comfort and a more mobility than usual BIVAD with their large and noisy displacement pump. Further studies are however necessary in order to best define the role of this innovative MCS in the context of the BTT strategy.
Conclusions
Long-term continuous-flow LVAD are an effective treatment option to bridge for a variable time period to cardiac transplantation patients scheduled on waiting list as outlined by a constant improvement over time in terms of better functional status, quality of life and survival, reduced adverse events and proven devices durability and reliability. Patients supported with these devices experience enhanced waitlist survival and have post-transplant survival rates comparable to that of conventional cardiac transplantation. In the next future the resolution of ongoing issues such as patients’ selection and management and optimal timing of LVAD implantation and transplantation, the continuous fight to reduce the rate of adverse events including bleeding, infection and stroke and the routine use of continuous-flow BIVAD will participate to further improve the results of the BTT strategy.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Lund LH, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Official Adult Heart Transplant Report--2013; focus theme: age. J Heart Lung Transplant 2013;32:951-64. [PubMed]
- Kirklin JK, Naftel DC, Pagani FD, et al. Sixth INTERMACS annual report: a 10,000-patient database. J Heart Lung Transplant 2014;33:555-64. [PubMed]
- Pagani FD. Continuous-flow rotary left ventricular assist devices with "3rd generation" design. Semin Thorac Cardiovasc Surg 2008;20:255-63. [PubMed]
- John R. Current axial-flow devices--the HeartMate II and Jarvik 2000 left ventricular assist devices. Semin Thorac Cardiovasc Surg 2008;20:264-72. [PubMed]
- Griffith BP, Kormos RL, Borovetz HS, et al. HeartMate II left ventricular assist system: from concept to first clinical use. Ann Thorac Surg 2001;71:S116-20; discussion S114-6.
- Frazier OH, Delgado RM 3rd, Kar B, et al. First clinical use of the redesigned HeartMate II left ventricular assist system in the United States: a case report. Tex Heart Inst J 2004;31:157-9. [PubMed]
- Frazier OH, Gemmato C, Myers TJ, et al. Initial clinical experience with the HeartMate II axial-flow left ventricular assist device. Tex Heart Inst J 2007;34:275-81. [PubMed]
- Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med 2007;357:885-96. [PubMed]
- Pagani FD, Miller LW, Russell SD, et al. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol 2009;54:312-21. [PubMed]
- Starling RC, Naka Y, Boyle AJ, et al. Results of the post-U.S. Food and Drug Administration-approval study with a continuous flow left ventricular assist device as a bridge to heart transplantation: a prospective study using the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support). J Am Coll Cardiol 2011;57:1890-8. [PubMed]
- John R, Kamdar F, Eckman P, et al. Lessons learned from experience with over 100 consecutive HeartMate II left ventricular assist devices. Ann Thorac Surg 2011;92:1593-9; discussion 1599-600. [PubMed]
- Lok SI, Martina JR, Hesselink T, et al. Single-centre experience of 85 patients with a continuous-flow left ventricular assist device: clinical practice and outcome after extended support. Eur J Cardiothorac Surg 2013;44:e233-8. [PubMed]
- Westaby S, Banning AP, Jarvik R, et al. First permanent implant of the Jarvik 2000 Heart. Lancet 2000;356:900-3. [PubMed]
- Frazier OH, Gregoric ID, Delgado RM, et al. Initial experience with the Jarvik 2000 left ventricular assist system as a bridge to transplantation: report of 4 cases. J Heart Lung Transplant 2001;20:201. [PubMed]
- Frazier OH, Myers TJ, Gregoric ID, et al. Initial clinical experience with the Jarvik 2000 implantable axial-flow left ventricular assist system. Circulation 2002;105:2855-60. [PubMed]
- Frazier OH, Myers TJ, Westaby S, et al. Use of the Jarvik 2000 left ventricular assist system as a bridge to heart transplantation or as destination therapy for patients with chronic heart failure. Ann Surg 2003;237:631-6; discussion 636-7. [PubMed]
- Siegenthaler MP, Frazier OH, Beyersdorf F, et al. Mechanical reliability of the Jarvik 2000 Heart. Ann Thorac Surg 2006;81:1752-8; discussion 1758-9.
- Haj-Yahia S, Birks EJ, Rogers P, et al. Midterm experience with the Jarvik 2000 axial flow left ventricular assist device. J Thorac Cardiovasc Surg 2007;134:199-203. [PubMed]
- Sorensen EN, Pierson RN 3rd, Feller ED, et al. University of Maryland surgical experience with the Jarvik 2000 axial flow ventricular assist device. Ann Thorac Surg 2012;93:133-40. [PubMed]
- Strueber M, O'Driscoll G, Jansz P, et al. Multicenter evaluation of an intrapericardial left ventricular assist system. J Am Coll Cardiol 2011;57:1375-82. [PubMed]
- Aaronson KD, Slaughter MS, Miller LW, et al. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation 2012;125:3191-200. [PubMed]
- Slaughter MS, Pagani FD, McGee EC, et al. HeartWare ventricular assist system for bridge to transplant: combined results of the bridge to transplant and continued access protocol trial. J Heart Lung Transplant 2013;32:675-83. [PubMed]
- Strueber M, Larbalestier R, Jansz P, et al. Results of the post-market Registry to Evaluate the HeartWare Left Ventricular Assist System (ReVOLVE). J Heart Lung Transplant 2014;33:486-91. [PubMed]
- Wieselthaler GM. Initial clinical experience with a novel left ventricular assist device with a magnetically levitated rotor in a multi-institutional trial. J Heart Lung Transplant 2010;29:1218-25. [PubMed]
- Najjar SS, Slaughter MS, Pagani FD, et al. An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. J Heart Lung Transplant 2014;33:23-34. [PubMed]
- Morshuis M, El-Banayosy A, Arusoglu L, et al. European experience of DuraHeart magnetically levitated centrifugal left ventricular assist system. Eur J Cardiothorac Surg 2009;35:1020-7; discussion 1027-8. [PubMed]
- Moazami N, Steffen RJ, Naka Y, et al. Lessons learned from the first fully magnetically levitated centrifugal LVAD trial in the United States: the DuraHeart trial. Ann Thorac Surg 2014;98:541-7. [PubMed]
- Frazier OH, Rose EA, Oz MC, et al. Multicenter clinical evaluation of the HeartMate vented electric left ventricular assist system in patients awaiting heart transplantation. J Thorac Cardiovasc Surg 2001;122:1186-95. [PubMed]
- Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001;345:1435-43. [PubMed]
- Dembitsky WP, Tector AJ, Park S, et al. Left ventricular assist device performance with long-term circulatory support: lessons from the REMATCH trial. Ann Thorac Surg 2004;78:2123-9; discussion 2129-30. [PubMed]
- John R, Kamdar F, Liao K, et al. Improved survival and decreasing incidence of adverse events with the HeartMate II left ventricular assist device as bridge-to-transplant therapy. Ann Thorac Surg 2008;86:1227-34. [PubMed]
- John R, Kamdar F, Liao K, et al. Improved survival and decreasing incidence of adverse events with the HeartMate II left ventricular assist device as bridge-to-transplant therapy. Ann Thorac Surg 2008;86:1227-34; discussion 1234-5. [PubMed]
- Westaby S, Jarvik R, Freeland A, et al. Postauricular percutaneous power delivery for permanent mechanical circulatory support. J Thorac Cardiovasc Surg 2002;123:977-83. [PubMed]
- Deuse T, Reichenspurner H. Do not touch the sternum--thoracotomy incisions for HVAD implantation. ASAIO J 2014;60:234-6. [PubMed]
- Russell SD, Rogers JG, Milano CA, et al. Renal and hepatic function improve in advanced heart failure patients during continuous-flow support with the HeartMate II left ventricular assist device. Circulation 2009;120:2352-7. [PubMed]
- Rogers JG, Aaronson KD, Boyle AJ, et al. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol 2010;55:1826-34. [PubMed]
- McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787-847. [PubMed]
- John R, Pagani FD, Naka Y, et al. Post-cardiac transplant survival after support with a continuous-flow left ventricular assist device: impact of duration of left ventricular assist device support and other variables. J Thorac Cardiovasc Surg 2010;140:174-81. [PubMed]
- Kamdar F, John R, Eckman P, et al. Postcardiac transplant survival in the current era in patients receiving continuous-flow left ventricular assist devices. J Thorac Cardiovasc Surg 2013;145:575-81. [PubMed]
- Deo SV, Sung K, Daly RC, et al. Cardiac transplantation after bridged therapy with continuous flow left ventricular assist devices. Heart Lung Circ 2014;23:224-8. [PubMed]
- Donneyong M, Cheng A, Trivedi JR, et al. The association of pretransplant HeartMate II left ventricular assist device placement and heart transplantation mortality. ASAIO J 2014;60:294-9. [PubMed]
- Taghavi S, Jayarajan SN, Komaroff E, et al. Continuous flow left ventricular assist device technology has influenced wait times and affected donor allocation in cardiac transplantation. J Thorac Cardiovasc Surg 2014;147:1966-71, 1971.e1.
- Delgado JF, Gómez-Sánchez MA, Sáenz de la Calzada C, et al. Impact of mild pulmonary hypertension on mortality and pulmonary artery pressure profile after heart transplantation. J Heart Lung Transplant 2001;20:942-8. [PubMed]
- Kirklin JK, Naftel DC, Bourge RC, et al. Evolving trends in risk profiles and causes of death after heart transplantation: a ten-year multi-institutional study. J Thorac Cardiovasc Surg 2003;125:881-90. [PubMed]
- Gallagher RC, Kormos RL, Gasior T, et al. Univentricular support results in reduction of pulmonary resistance and improved right ventricular function. ASAIO Trans 1991;37:M287-8. [PubMed]
- Adamson RM, Dembitsky WP, Jaski BE, et al. Left ventricular assist device support of medically unresponsive pulmonary hypertension and aortic insufficiency. ASAIO J 1997;43:365-9. [PubMed]
- Martin J, Siegenthaler MP, Friesewinkel O, et al. Implantable left ventricular assist device for treatment of pulmonary hypertension in candidates for orthotopic heart transplantation-a preliminary study. Eur J Cardiothorac Surg 2004;25:971-7. [PubMed]
- Salzberg SP, Lachat ML, von Harbou K, et al. Normalization of high pulmonary vascular resistance with LVAD support in heart transplantation candidates. Eur J Cardiothorac Surg 2005;27:222-5. [PubMed]
- Etz CD, Welp HA, Tjan TD, et al. Medically refractory pulmonary hypertension: treatment with nonpulsatile left ventricular assist devices. Ann Thorac Surg 2007;83:1697-705. [PubMed]
- John R, Liao K, Kamdar F, et al. Effects on pre- and posttransplant pulmonary hemodynamics in patients with continuous-flow left ventricular assist devices. J Thorac Cardiovasc Surg 2010;140:447-52. [PubMed]
- Pauwaa S, Bhat G, Tatooles AJ, et al. How effective are continuous flow left ventricular assist devices in lowering high pulmonary artery pressures in heart transplant candidates? Cardiol J 2012;19:153-8. [PubMed]
- Kutty RS, Parameshwar J, Lewis C, et al. Use of centrifugal left ventricular assist device as a bridge to candidacy in severe heart failure with secondary pulmonary hypertension. Eur J Cardiothorac Surg 2013;43:1237-42. [PubMed]
- Cleveland JC Jr, Naftel DC, Reece TB, et al. Survival after biventricular assist device implantation: an analysis of the Interagency Registry for Mechanically Assisted Circulatory Support database. J Heart Lung Transplant 2011;30:862-9. [PubMed]
- Kirsch M, Mazzucotelli JP, Roussel JC, et al. Survival after biventricular mechanical circulatory support: does the type of device matter? J Heart Lung Transplant 2012;31:501-8. [PubMed]
- Frazier OH, Myers TJ, Gregoric I. Biventricular assistance with the Jarvik FlowMaker: a case report. J Thorac Cardiovasc Surg 2004;128:625-6. [PubMed]
- Strueber M, Meyer AL, Malehsa D, et al. Successful use of the HeartWare HVAD rotary blood pump for biventricular support. J Thorac Cardiovasc Surg 2010;140:936-7. [PubMed]
- Hetzer R, Krabatsch T, Stepanenko A, et al. Long-term biventricular support with the heartware implantable continuous flow pump. J Heart Lung Transplant 2010;29:822-4. [PubMed]
- Loebe M, Bruckner B, Reardon MJ, et al. Initial clinical experience of total cardiac replacement with dual HeartMate-II axial flow pumps for severe biventricular heart failure. Methodist Debakey Cardiovasc J 2011;7:40-4. [PubMed]
- Saito S, Sakaguchi T, Miyagawa S, et al. Biventricular support using implantable continuous-flow ventricular assist devices. J Heart Lung Transplant. 2011;30:475-8. [PubMed]
- Krabatsch T, Potapov E, Stepanenko A, et al. Biventricular circulatory support with two miniaturized implantable assist devices. Circulation 2011;124:S179-86. [PubMed]


