The impact of concordance with a lung cancer diagnosis pathway guideline on treatment access in patients with stage IV lung cancer
Introduction
Lung cancer is the leading cause of cancer mortality worldwide (1). In 2016, there were 28,539 lung cancer diagnoses in Canada with 20,800 lung cancer deaths, representing a 73% mortality (2). The majority of lung cancers are diagnosed at an advanced stage which is responsible for a poor 5-year lung cancer survival (~16%) (3). In Ontario, of staged cancers (prostate, female breast, colorectal, lung and cervix), lung cancer is most likely to be diagnosed at stage III or IV (70.5% of all staged lung cancers) with stage IV cancers accounting for 49.4% of all staged lung cancers (4). Diagnosis of stage IV lung cancer is associated with poor survival and high health care utilization (5). A large proportion of Ontario patients with advanced lung cancer are hospitalized in the final 30 days of life which may reflect gaps in addressing needs of this patient population (4).
An efficient approach to the assessment and diagnosis of patients with suspected lung cancer is essential in providing prompt access to effective treatment. However, delays in this process are commonly encountered with significant heterogeneity observed across healthcare institutions (6). Such delays may be due to prolonged diagnostic work up preceding and following specialist referral (7).
In response to the need for an efficient and multidisciplinary approach to the assessment of patients with suspected lung cancer, tertiary health institutions within Ontario have established streamlined diagnostic assessment pathways (8,9). One such program was effective in reducing the time from initial suspicion of lung cancer to a diagnosis being made (8). This improvement was achieved by a multifaceted approach including a streamlined referral process and a common diagnostic algorithm.
Similarly, the University Health Network (UHN) established the Lung Rapid Assessment and Management Program (LungRAMP) in 2009. This multidisciplinary Diagnostic Assessment Program (DAP) aimed to streamline and improve access to cancer care in Ontario lung cancer patients. Between 2010 and 2017, the UHN DAP has seen a 60% increase in referrals. Overall, 2,700 patients were assessed. The majority of referred cancer patients (70%) were not surgical candidates. Advanced lung cancer has a negative impact on patient’s quality of life (QOL) and survival (10) and is associated with high health care-related costs (11-13). Timely access to treatment is dependent on efficient and appropriate patient assessment and early referral for diagnostic workup. The UHN DAP has a 21-day target from receiving a patient referral to a decision to treat, which has been based on the expert consensus within Cancer Care Ontario (CCO) with a goal to have at least 50% of patients referred to any lung cancer DAP, meet this arbitrary target (14).
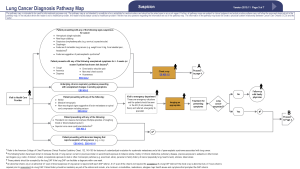
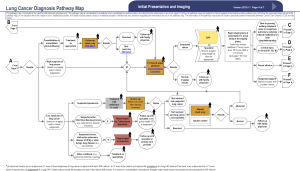
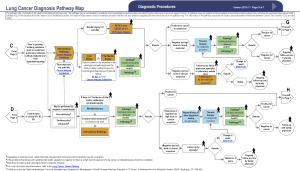
In November of 2015 a multidisciplinary expert panel assembled by the CCO updated the existing Lung Cancer Pathway Map, to serve as an evidence-based best practice guide to diagnostic assessment of patients with suspected and known lung cancer (15). This utility of this new Lung Cancer Diagnostic Pathway Guideline (LCDPG) in improving patient access to prompt diagnosis and treatment has not previously been assessed in clinical practice.
This study aims to assess the impact of referral concordance with a new LCDPG on access to treatment in patients with stage IV lung cancer. It also aims to assess the efficiency of a DAP in providing a diagnosis and access to treatment in patients with stage IV lung cancer. We present the following article in accordance with the STROBE reporting checklist.
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization. Ethics approval for this study was granted by the Research Ethics Board (REB) at the UHN. A single center retrospective cohort study was conducted of adult patients referred to the UHN DAP with a clinical stage IV lung cancer between November 1, 2015 and May 31, 2017. Lung cancer staging was based on the 7th edition of the TNM staging system (16). Existing medical records were retrospectively analyzed. These included province wide electronic medical records (Connecting Ontario), the UHN electronic patient record (EPR) and archived patient referral documents.
Patients were excluded if they received a diagnosis of malignancy other than lung cancer or if a definitive tissue diagnosis was not reached. Patients were also excluded if clinical stage at the time of diagnosis was not stage IV. Patients meeting the inclusion criteria were divided into two groups: (I) patients referred in concordance with the CCO LCDPG; (II) patients referred in discordance with the CCO LCDPG. Initial healthcare assessment and referral was deemed discordant according to the following categories: (I) inadequate initial investigation of suspicious symptoms as per CCO LCDPGs (15); (II) incorrect follow-up of presumed alternative diagnosis as per CCO LCDPGs; (III) no further imaging for suspicious chest radiograph; (IV) no referral made after suspicious CT imaging as per CCO LCDPGs.
The primary study outcome was time from initial healthcare presentation to treatment initiation. Secondary outcomes were (I) time from initial healthcare presentation to diagnosis; (II) time from initial healthcare presentation to decision to treat; (III) time from referral to treatment; (IV) time from referral to diagnostic procedure; (V) time from referral to decision to treat; (VI) healthcare utilisation from referral to treatment initiation; (VII) number of diagnostic procedures required to achieve a diagnosis; and (VIII) type of diagnostic procedure used.
The initial healthcare presentation was defined as the first documented episode of the patient being assessed for signs, symptoms, or investigation findings attributable to the eventual diagnosis of lung cancer. The date of decision to treat was defined as the date on which an initial treatment modality was chosen, and/or a referral to an appropriate oncology service was made for consideration of treatment.
Statistical analysis
Descriptive statistics were summarized as either mean (± SD), 95% CI or median with corresponding Interquartile range (IQR) for continuous data (depending on its distribution), or frequencies for categorical data. Comparisons between patient groups were assessed using the Student t-test for continuous data, and Fisher’s Exact Test for categorical data. All tests were two sided with a P value of <0.05 indicating statistical significance. Analysis was conducted using Microsoft Excel (Microsoft Office 365 ProPlus Version 15.0.4911.1002).
Results
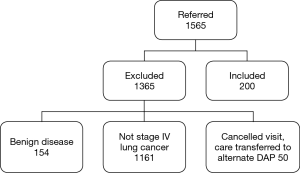
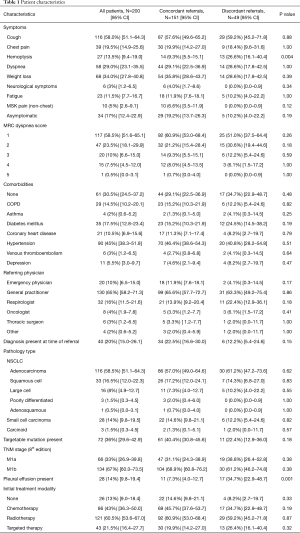
In the time frame of the study, 1,565 patients were referred to the UHN DAP. One thousand three hundred and sixty-five patients did not meet the inclusion criteria (Figure 1). A total of 200 patients met the inclusion criteria and were included in the final analysis. One hundred and fifty-one (75.5%) patients were assessed and referred in concordance with the CCO guidelines. Discordant assessment and referral occurred in 49 patients (24.5%). Of these discordant referrals, the reasons for discordance included inadequate initial investigation of suspicious symptoms (32.7%), incorrect follow-up of presumed alternative diagnosis (32.7%), no further imaging for suspicious chest radiograph (24.5%), and no referral to DAP despite suspicious CT imaging (30.6%). Clinical characteristics of all patients are outlined in Table 1. Patient characteristics were similar in both groups however the frequency of hemoptysis was higher in patients referred discordantly (concordant 9.3% vs. discordant 26.6%, P=0.004). The presence of pleural effusion was also more common in discordant referrals (7.3% vs. 34.7%, P=0.001). Forty patients (20%) were referred to the DAP after a tissue diagnosis was made.
Full table
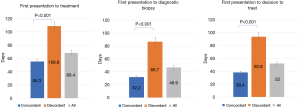
The impact of referral concordance with CCO guidelines on patient treatment access from first healthcare presentation is outlined in Figure 2. Time from first presentation to treatment was significantly shorter in patients referred in concordance with CCO guidelines compared with discordant referrals (55.3±3.1 vs. 108.8±5.0 days, P<0.001). Time from first presentation to diagnostic biopsy (32.2±1.8 vs. 86.7±6.9 days, P<0.001) and time from first presentation to decision to treat (38.4±1.9 vs. 93.8±6.5 days, P<0.001) were also significantly reduced when referrals were concordant with guidelines. Time from healthcare presentation to treatment was not significantly different between patients referred to the DAP with or without a tissue diagnosis (68.4±46.6 vs. 68.5±46.8 days, P=0.99)
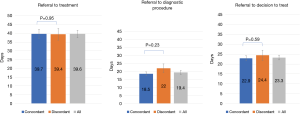
The impact of referral concordance on treatment access from time of referral is displayed in Figure 3. There was no significant difference between concordant and discordant referrals in time from referral to diagnostic biopsy (18.5±1.2 vs. 22.0±2.7 days, P=0.23), decision to treat (22.9±1.3 vs. 24.4±2.6 days, P=0.59), or treatment initiation (39.7±2.6 vs. 39.4±3.3 days, P=0.95). Decision to treat was within 21 days of referral in 54.5% of all patients referred to the DAP. An average of 4.9 patient visits to hospital occurred between referral to DAP and treatment initiation. There was no significant difference observed in hospital visits between concordant and discordant referrals.
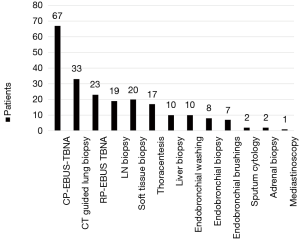
Diagnostic practices in patients referred with stage IV lung cancer are outlined in Table 2. Ninety one percent of patients referred required only one diagnostic procedure. There was no difference observed between concordant and discordant referrals in the number of diagnostic procedures required. The most common diagnostic procedure used was endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) 33.5% (95% CI, 27.3–40.3) (Figure 4). The most common location for performing diagnostic procedures was the endoscopy suite [49.0% (95% CI, 42.2–55.9)].

Full table
Discussion
Lung cancer is associated with significant morbidity and mortality with the majority of patients presenting with advanced disease (3). Prompt diagnostic assessment is essential to enable timely access to appropriate treatment modalities (14). The revised CCO LCDPG provides a framework to guide initial patient assessment and referral to a DAP for ongoing management (15). Our study confirms that concordance with this pathway in referring patients to a DAP reduces time to treatment access. Specifically, initial guideline concordant assessment and subsequent DAP referral was associated with reduced time from initial healthcare presentation to treatment compared with discordant referrals. Time from initial healthcare presentation to diagnosis and decision to treat were also reduced with guideline concordance.
Despite the delay in accessing treatment seen in patients assessed and referred in a discordant manner, it is encouraging that the majority of patients (75.5%) were referred in concordance with the guidelines. There was no difference observed in the referral practices of General Practitioners and Specialist Physicians with regards to the CCO guidelines with similar levels of concordance seen. The specific reason for guideline discordance was varied amongst patients. The most common issue that resulted in discordant initial assessment was inadequate investigation of suspicious symptoms in a high-risk patient as defined by the CCO guidelines (see Supplementary). The most frequently encountered example of this was an unexplained cough for greater than 3 weeks that was not investigated with a chest X-ray as recommended by these guidelines. Other issues were also encountered to a similar degree, including suspicious chest radiographs or CT imaging not being further investigated or referred to the DAP as per CCO guidelines. More than one reason for discordance was also seen in some patients.
Pleural effusions and hemoptysis were more frequently observed in patients initially assessed and referred discordantly. The exact reason for this difference is not completely clear. It is possible that the delay in referral of these patients may have resulted in further disease progression leading to these findings. However, this conclusion can only be made based on the assumption that the majority of patients initially presented to a healthcare provider with similar levels of disease burden which cannot be known from this study. Alternatively, it is possible that these findings might have been originally attributed to other medical issues (i.e., bronchitis or pneumonia for patient presenting with hemoptysis) and managed accordingly, before considering a possibility of lung cancer and pursuing appropriate lung cancer DAP referral. It is important however to reinforce that prompt patient referral and diagnosis enables timely access to treatment which may potentially reduce the frequency of such disease manifestations that impact morbidity, mortality, and healthcare utilization (5). Further studies are needed to test this hypothesis.
Time from patient referral to treatment initiation was not influenced by concordance with referral guidelines. This outcome was expected as the impact of discordance with initial assessment and referral guidelines is observed in the time period prior to a DAP referral being made. As such, the delay in access to treatment is accounted for by a delay in the time from initial healthcare presentation to the time of referral. Therefore, once a referral was received by the DAP, all patients appear to have been assessed and managed by the DAP in a similar manner resulting in no significant differences in access to treatment.
Our study supports the hypothesis that a streamlined diagnostic assessment pathway is effective in providing prompt treatment access to patients with stage IV lung cancer (6,7). Overall, a decision to treat was made within 21 days from the time of referral in 54.5% of patients, satisfying the arbitrary CCO target of 50%. Adequate tissue was obtained with a single diagnostic procedure in the vast majority of patients (91.0%). Approximately half of the diagnostic procedures completed were performed in the endoscopy suite via bronchoscopy with EBUS-TBNA being the most common method used. It is important to note however that a significant number of patients were able to undergo a diagnostic procedure in an outpatient clinic setting. This included performing diagnostic thoracentesis, peripheral lymph node biopsy, soft tissue biopsy, and sputum sampling. The use of these diagnostic techniques has multiple advantages. Firstly, the risk of complication is generally lower when performing these procedures compared with endoscopic or surgical procedures which require general anesthesia or deep sedation (17,18). Radiology guided procedures also may carry a higher risk of complication such as pneumothorax from percutaneous lung biopsy (19). Time to diagnosis can also be reduced by performing diagnostic procedures in the outpatient clinic setting at the time of the original consultation by eliminating the need for an alternative procedure that requires a subsequent hospital visit at a later date. Finally, healthcare cost can be reduced by performing procedures in the outpatient setting that require less hospital resources (20,21). Over the years our DAP has focused on streamlining the diagnostic assessment of patients with thoracic malignancy with focus on ambulatory care. With opening of the Interventional Thoracic Surgery Suite (ITSS), many of the diagnostic procedures required in patients with lung cancer performed routinely in the operating room, have been transferred to the ambulatory setting. This resulted in a reduced health care utilization and costs without compromise to diagnostic yield (20,21).
CCO guidelines recommend a 21-day target for a treatment decision to be made in all patients referred with a new diagnosis of lung cancer. These consensus guidelines were based on recommendations from the Canadian Society of Surgical Oncology and the CCO expert consensus. This decision to treat target is based primarily on evidence relating to patients with lung cancer potentially curable with either surgery or radiotherapy (22,23). As such, the predominant concern affecting wait times in these patients is the rate of tumor growth and development of metastasis which may result in the development of more advanced or incurable disease (24,25). In patients with incurable stage IV disease at the time of referral, reasons for prompt treatment may differ. For example, patients with advanced stage lung cancer are more likely to present with significant symptoms than patients with more limited disease who may be asymptomatic (25). As a result, initiation of treatment in advanced stage lung cancer may be required more urgently for symptom palliation, particularly in cases of severe or life-threatening symptoms such as hemoptysis, spinal cord compression, or superior vena cava obstruction (25). Therefore, consideration should be made as to whether the existing CCO guidelines regarding decision to treat and treatment initiation targets for lung cancer should be stratified according to stage and symptom burden. Healthcare utilization may also differ between patients with stage IV lung cancer and less advanced disease, during the initial assessment period. Our study did not directly assess this and as such further research regarding this possibility may be warranted.
The majority of patients (80%) were referred to the DAP without a tissue diagnosis. Of significance, in patients where a tissue diagnosis was made prior to referral, the time to treatment was not significantly shorter than those patients referred without a tissue diagnosis. Therefore, despite the perceived advantage of making a diagnosis prior to DAP referral, access to treatment is not improved as a result. The reasons for this are unclear. Potentially this may represent the need for such patients to still undergo further investigations to complete staging (to confirm the suspected stage IV disease) that may have not been done prior to referral. These investigations may be non-invasive such as FDG-PET imaging or may be invasive to confirm the presence of distant disease by direct tissue sampling. As such, some patients may require further invasive diagnostic procedures despite a diagnosis of lung cancer being made prior to referral. Therefore, we would suggest that patients with a suspected clinical stage IV lung cancer be referred to a DAP to both streamline assessment and limit invasive diagnostic procedures to a minimum.
While non-invasive investigations are an important component of the diagnostic and staging algorithm of lung cancer, invasive diagnostic procedures are still required in the majority of patients to guide management decisions. This requirement has traditionally exposed patients to procedures associated with significant morbidity and healthcare burden (26). However, in recent years, minimally invasive procedures are increasingly becoming the standard of care (27). EBUS-TBNA is one such example of a minimally invasive technique that enables high diagnostic accuracy while reducing complications and morbidity associated with more traditional methods such as mediastinoscopy (28-31). The development of newer targeted therapies for lung cancer has resulted in the need to perform adequate subtyping and molecular testing on diagnostic specimens. Updated molecular testing guidelines for the selection of lung cancer patients for treatment with novel targeted therapies now recommend an extensive panel of ancillary testing to be performed on diagnostic material (32). Again, minimally invasive techniques such as EBUS-TBNA have been shown to be adequate in obtaining sufficient material for this assessment (33-37). In this study, 36% of patients with stage IV lung cancer were found to have an activating mutation or molecular characteristic potentially suitable for novel targeted therapies. As a result, 21.5% of patients were commenced on targeted therapy as their initial treatment. Importantly, in those patients found to have a targetable mutation, the vast majority (93.1%) were able to be identified with a single diagnostic procedure. It is also noteworthy that cytology specimens from minimally invasive techniques performed in the endoscopy suite and outpatient clinic were used for the majority of patients who had a targetable mutation identified.
Our study has several limitations which predominantly relate to its retrospective design. Methodology relied on existing electronic and paper medical records. Specifically, the date of initial healthcare presentation for each patient was determined from archived referrals, physician reports, and electronic records which may have not encompassed earlier presentations to a healthcare provider. Similarly, defining referral concordance was based on retrospective records. Also, although all patient visits in our institution were able to be readily analyzed, encounters with external healthcare providers in the community and in other medical facilities may not have been documented in the online systems available for review. This may have affected our data assessing the number of healthcare visits between DAP referral and treatment initiation which did not differ between discordant and concordant referrals. Despite this, we believe these factors did not significantly affect the validity of our data and primary outcome as each patient group would be equally affected by these limitations.
In conclusion, concordance with a lung cancer diagnostic assessment pathway guideline improves access to treatment in patients with stage IV lung cancer. This study also demonstrates that a lung cancer DAP is effective in providing a streamlined pathway to prompt diagnosis and treatment. The use of such guidelines and DAPs should be promoted and used to guide the assessment and referral patients with advanced lung cancer. Future research and education should focus on improving factors that delay DAP referral.
Acknowledgments
Funding: None.
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-157
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-157). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization. Ethics approval for this study was granted by the Research Ethics Board (REB) at the University Health Network (UHN) (CAPCR ID: 17-5642.2). As this was a retrospective cohort study, not involving human experiments, waiver of consent was approved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization. Cancer: fact sheet. World Health Organization, 2017. Available online: http://www.who.int/news-room/fact-sheets/detail/cancer
- Canadian Cancer Society. Canadian Cancer Statistics 2016. In: Canadian Cancer Society, 2016. Available online: http://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/Canadian-Cancer-Statistics-2016-EN.pdf?la=en
- Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med 2011;32:605-44. [Crossref] [PubMed]
- Cancer Care Ontario. Ontario Cancer Statistics. In: Cancer Care Ontario, 2016.
- Bremner KE, Krahn MD, Warren JL, et al. An international comparison of costs of end-of-life care for advanced lung cancer patients using health administrative data. Palliat Med 2015;29:918-28. [Crossref] [PubMed]
- Jacobsen MM, Silverstein S, Quinn M, et al. Timeliness of access to lung cancer diagnosis and treatment: A scoping literature review. Lung Cancer 2017;112:156-64. [Crossref] [PubMed]
- Liberman M, Liberman D, Sampalis JS, et al. Delays to surgery in non-small cell lung cancer. Can J Surg 2006;49:31-6. [PubMed]
- Lo DS, Zeldin R, Skrastins R, et al. Time to Treat: A System Redesign Focusing on Decreasing the Time from Suspicion of Lung Cancer to Diagnosis. J Thorac Oncol 2007;2:1001-6. [Crossref] [PubMed]
- Dahele M, Ung Y, Meharchand J, et al. Integrating regional and community lung cancer services to improve patient care. Curr Oncol 2007;14:234-7. [Crossref] [PubMed]
- Evans WK, Connor Gorber S, Spence ST, et al. Health State descriptions for Canadians: Cancers. In. Canada Statistics Canada 2005. Available online: http://publications.gc.ca/collections/Collection/Statcan/82-619-MIE/82-619-MIE2005001.pdf
- Yabroff KR, Lund J, Kepka D, et al. Economic burden of cancer in the United States: estimates, projections, and future research. Cancer Epidemiol Biomarkers Prev 2011;20:2006-14. [Crossref] [PubMed]
- Warren JL, Barbera L, Bremner KE, et al. End-of-life care for lung cancer patients in the United States and Ontario. J Natl Cancer Inst 2011;103:853-62. [Crossref] [PubMed]
- Yabroff KR, Warren JL. High-cost imaging in elderly patients with stage IV cancer: challenges for research, policy, and practice. J Natl Cancer Inst 2012;104:1113-4. [Crossref] [PubMed]
- Darling GE, Maziak DE, Clifton JC, et al. The practice of thoracic surgery in Canada. Can J Surg 2004;47:438-45. [PubMed]
- Available online: https://www.cancercareontario.ca/en/pathway-maps/lung-cancer
- Mirsadraee S, Oswal D, Alizadeh Y, et al. The 7th lung cancer TNM classification and staging system: Review of the changes and implications. World J Radiol 2012;4:128-34. [Crossref] [PubMed]
- Ault MJ, Rosen B, Scher J, et al. Thoracentesis outcomes: a 12-year experience. Thorax 2015;70:127-32. [Crossref] [PubMed]
- Łochowski MP, Kozak J. Video-assisted thoracic surgery complications. Wideochir Inne Tech Maloinwazyjne 2014;9:495-500. [Crossref] [PubMed]
- Manhire A, Charig M, Clelland C, et al. Guidelines for radiologically guided lung biopsy. Thorax 2003;58:920-36. [Crossref] [PubMed]
- Czarnecka-Kujawa K, Rochau U, Siebert U, et al. Cost-effectiveness of mediastinal lymph node staging in non-small cell lung cancer. J Thorac Cardiovasc Surg 2017;153:1567-8. [Crossref] [PubMed]
- McDonald CM, Pierre C, de Perrot M, et al. Efficacy and Cost of Awake Thoracoscopy and Video-Assisted Thoracoscopic Surgery in the Undiagnosed Pleural Effusion. Ann Thorac Surg 2018;106:361-7. [Crossref] [PubMed]
- O'Rourke N, Edwards R. Lung cancer treatment waiting times and tumour growth. Clin Oncol (R Coll Radiol) 2000;12:141-4. [Crossref] [PubMed]
- Christensen ED, Harvald T, Jendresen M, et al. The impact of delayed diagnosis of lung cancer on the stage at the time of operation. Eur J Cardiothorac Surg 1997;12:880-4. [Crossref] [PubMed]
- Mohammed N, Kestin L, Grills I, et al. Rapid disease progression with delay in treatment of non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2011;79:466-72. [Crossref] [PubMed]
- Ost DE, Sai-Ching J, Tanoue L, et al. Clinical and Organisational Factors in Initial Evaluation of Patients With Lung Cancer. Chest 2013;143:e121S-e141S. [Crossref] [PubMed]
- Ost DE, Niu J, Elting L, et al. Quality Gaps and Comparative Effectiveness in Lung Cancer Staging and Diagnosis. Chest 2014;145:331-45. [Crossref] [PubMed]
- Folch E, Costa D, Wright J, et al. Lung cancer diagnosis and staging in the minimally invasive age with increasing demands for tissue analysis. Transl Lung Cancer Res 2015;4:392-403. [PubMed]
- Yasufuku K, Chiyo M, Koh E, et al. Endobronchial ultrasound guided transbronchial needle aspiration for staging of lung cancer. Lung Cancer 2005;50:347-54. [Crossref] [PubMed]
- Navani N, Nankivell M, Lawrence DR. Lung cancer diagnosis and staging with endobronchial ultrasound-guided transbronchial needle aspiration compared with conventional approaches: an open-label, pragmatic, randomised controlled trial. Lancet Respir Med 2015;3:282-9. [Crossref] [PubMed]
- Czarnecka-Kujawa K, Yasufuku K. The role of endobronchial ultrasound versus mediastinoscopy for non-small cell lung cancer. J Thorac Dis 2017;9:S83-S97. [Crossref] [PubMed]
- Ernst A, Anantham D, Eberhardt R, et al. Diagnosis of Mediastinal Adenopathy—Real-Time Endobronchial Ultrasound Guided Needle Aspiration versus Mediastinoscopy. J Thorac Oncol 2008;3:577-82. [Crossref] [PubMed]
- Lindeman NI, Cagle PT, Aisner DL, et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch Pathol Lab Med 2018;142:321-46. [Crossref] [PubMed]
- Casadio C, Guarize J, Donghi S, et al. Molecular testing for targeted therapy in advanced non-small cell lung cancer: suitability of endobronchial ultrasound transbronchial needle aspiration. Am J Clin Pathol 2015;144:629-34. [Crossref] [PubMed]
- Billah S, Stewart J, Staerkel G, et al. EGFR and KRAS mutations in lung carcinoma: molecular testing by using cytology specimens. Cancer Cytopathol 2011;119:111-7. [Crossref] [PubMed]
- Lewandowska MA, Jozwicki W, Jochymski C, et al. Application of PCR methods to evaluate EGFR, KRAS and BRAF mutations in a small number of tumor cells in cytological material from lung cancer patients. Oncol Rep 2013;30:1045-52. [Crossref] [PubMed]
- Nakajima T, Yasufuku K, Nakagawara A, et al. Multigene mutation analysis of metastatic lymph nodes in non-small cell lung cancer diagnosed by endobronchial ultrasound-guided transbronchial needle aspiration. Chest 2011;140:1319-24. [Crossref] [PubMed]
- Lee JM, Heymann JJ, Pagan C, et al. Feasibility Of Pd-L1 Expression Testing In Non-Small Cell Lung Cancer From Ebus-Tbna Samples. Am J Respir Crit Care Med 2017;195:A2883.