Pleural controversies: image guided biopsy vs. thoracoscopy for undiagnosed pleural effusions?
Introduction
There are approximately 320 new cases of pleural effusions per 100,000 people each year in industrialised countries and their investigation and management provide a significant work load for respiratory and acute medical departments (1,2). The aetiology of a pleural effusion varies according to geographical location and population demographics. The investigation and treatment of patients with pleural effusions is now governed by several national guidelines and diagnostic algorithms (3,4). A definitive diagnosis is obtained either by thoracocentesis and analysis of pleural fluid cytology, or histological analysis of tissue obtained via surgical biopsy, image-guided biopsy or local anaesthetic thoracoscopy (LAT).
In the USA there are approximately 1.5 million new pleural effusions diagnosed each year (5). There are over 50 causes of pleural effusions, even though a small number of diagnoses are responsible for the vast majority of effusions (3). Traditionally, the causes of effusions can be categorized into transudative or exudative using Light’s criteria (6). Effusions secondary to heart, liver or renal failure are often transudative, whereas those caused by malignancy or infection are typically exudative. Malignancy, heart failure, tuberculosis (TB) and other respiratory infections are responsible for the vast majority of pleural effusions (5,7).
Malignant pleural effusions affect up to 15% of patients who die with malignancy (8). Lung and breast cancer are responsible for 50-65% of all malignant pleural effusions (9). An important subset of malignant diagnoses is mesothelioma, a malignant tumour of the pleura caused predominantly by asbestos exposure. Currently in the UK one person dies of mesothelioma every 4 hours and the incidence worldwide is increasing (9). The UK incidence is set to increase to a peak of 2,450 cases per year between 2011 and 2015 (10). Worldwide mesothelioma is expected to have caused 90,000 deaths by the year 2050.
One of the cornerstones of investigating the cause of a pleural effusion is thoracocentesis and cytological examination of pleural fluid. Pleural fluid cytology is only diagnostic for malignancy in approximately 60% of cases with a second sample only increasing this yield slightly (11). Cytology is often combined with cross sectional imaging to identify abnormal features which will help guide further investigation. Following cross-sectional imaging a tissue diagnosis is usually sought to confirm diagnosis and guide further management.
Obtaining diagnostic tissue can be achieved by surgical video assisted thoracic surgery (VATS), LAT or image-guided biopsy [computed tomography (CT) or ultrasonography assisted], with each technique having its own advantages and disadvantages. The diagnostic accuracy, patient acceptability, contraindications and complications are taken into account to enable careful selection of the appropriate investigation.
Historical aspects
Blind “closed needle” pleural biopsy with an Abrams or Cope needle has long been popular due to its relative inexpensiveness and practicality. The technique has been used since 1958 when it was proposed as a less invasive option to open pleural biopsy (12).
Thoracoscopy has its origins in 19th century Europe. Samuel Gordon published an article in 1866 in which he described monitoring the treatment of a young girl with empyema using a urological endoscope (13). It was almost 50 years later when Jacobaeus published the first case series of thoracoscopy, this time under local anaesthetic (14). He described in depth his technique, patient positioning and site of entry as well as its use in treating TB by collapsing infected lobes in a procedure that became eponymous. Following the advent of antimicrobials to treat TB the use of thoracoscopy declined until the 1980’s, when fibre optic and video technology led to a resurgence of both physician and surgeon led thoracoscopy. The provision of LAT services has dramatically increased in recent years largely due to its advantages as a diagnostic and therapeutic tool.
CT has been commercially available since the early 1970’s and is the gold standard for the acquisition of cross-sectional imaging of the pleura and pleural space. Reports of the diagnostic capabilities of CT-guided needle biopsy of pleural lesions have been published since the 1980s (15). US has been used since the first half of the 20th century when it was identified as a method of obtaining real-time images of abdominal masses (16). There are reports of ultrasound (US) being used to image the pleura dating back to the early 1960s when it was first described how fluid could be identified and sampled for diagnostic purposes (17). The use of thoracic US when undertaking pleural procedures has since become recommended best practice (1).
However, diagnostic thoracentesis and cytological analysis, rather than tissue biopsy, is often the first invasive procedure for patients presenting with pleural effusions.
“Tissue is the issue”
Pleural fluid cytology can provide diagnostic information in over half of patients with malignant pleural effusions (11). However, the diagnostic sensitivity depends upon the type of malignancy; adenocarcinoma, for instance, has a higher cytological detection rate than squamous cell carcinoma or lymphoma (3). In mesothelioma, only one in five cases are diagnosed by cytology alone (18). One experienced centre has reported a diagnostic rate of 73-76% for mesothelioma, with a subsequent reduction in the requirement for a tissue biopsy (19). However, this relied upon highly experienced cytologists who are unlikely to be readily available in all centres worldwide. Therefore, a high proportion of patients presenting with undiagnosed pleural effusions suspicious for malignancy require a pleural biopsy for confirmation of diagnosis. A definitive histological diagnosis and tumour receptor status not only aids the oncologist regarding possible targeted treatment options, but also provides important prognostic information for both patient and clinician (20).
Blind pleural biopsy
Blind or “closed” pleural biopsy is a technique whereby a needle, commonly a Cope or Abrams, is used to acquire pleural tissue under no direct pleural visualisation or real-time image guidance. In the last 15 years blind pleural biopsy has been shown to have a poor diagnostic yield in malignancy and its use is diminishing in many countries (21). TB, however, is one disease where blind biopsy remains an important diagnostic tool. Worldwide TB is an important cause of pleural effusions, though its prevalence is significantly different between countries. TB affects the pleura in a diffuse pattern allowing for a high sensitivity of blind biopsy of up to 80% when combined with pleural fluid culture (22,23). Given the relatively low cost and ease of accessibility of blind pleural biopsy, this technique will no doubt remain important in areas where the pre-test probability of TB is high.
US- and CT-guided biopsy
Image-guided biopsy performed under US or CT guidance allows focal pleural thickening or nodules to be biopsied accurately and safely. The choice between CT- and US-guided technique is dependent upon expertise, cost and equipment. US-guided biopsy allows for real time visualisation of the biopsy needle with no radiation risk to the patient. During US-guided biopsy patient movement due to heavy or rapid breathing in dyspnoeic patients can be compensated for in real time. The use of CT allows areas inaccessible to US to be biopsied (e.g., behind ribs). The size of the target lesions obviously dictates the ease of the procedure; however, pleural thickening as little as 5 mm has been effectively biopsied (24).
Technique
In CT-guided biopsy, preliminary images are acquired to determine the best site for biopsy whilst allowing for patient comfort. A limited CT scan then re-images the area of choice using intravenous contrast if necessary to identify an appropriate rib space for insertion of a biopsy needle. The needle is then advanced under local anaesthetic while limited CT scanning ensures that it is inserted into the area of interest. Once the sample is acquired the needle is removed. Post-biopsy haemorrhage or pneumothorax can then be excluded with a further limited CT scan immediately after the procedure, although the patient is observed for a further 4-6 hours during which they may have one or more plain chest radiographs to look for pneumothorax with a slow air leak (25). If there are no apparent complications and the patient is comfortable they can be discharged with advice on potential complications (25).
US-guided biopsy is usually carried out by a freehand technique whereby an area is identified using a low frequency curvilinear probe (2-5 mHz), the skin is marked and the biopsy is performed while the patient remains in the same position (26). Major blood vessels and viscera are identified prior to insertion of the needle to avoid complications. Inferior biopsy sites closer to the diaphragm have shown to be more likely to elicit positive biopsy samples as secondary metastases are most likely to be found here (27).
The choice of needle to use for biopsy has been under considerable interest in the past, the most commonly used needles being the cutting and the Tru-cut (28). Adams et al. found that a cutting needle biopsy was more sensitive than a fine needle aspiration in the diagnosis of malignancy including mesothelioma (24,29). The use of a larger cutting needle (14 vs. 18 gauge) has been shown to be of no diagnostic benefit (24,30). It has been proposed that combining both methods can lead to a higher overall diagnostic yield.
Advantages
There are several scenarios where image-guided biopsy has a clear benefit over thoracoscopy. Pleural thickening is not always accompanied by fluid in the pleural space; malignancy and certain other pleural diseases can cause fusion of the visceral and parietal pleurae preventing fluid accumulation (31). Image-guided biopsy does not require an effusion to ensure safety. However, a lack of fluid inherently increases the risk of pneumothorax secondary to lung perforation.
US-guided biopsy is cheap and relatively accessible and requires minimal consumables. Biopsies can be carried out by a suitably trained physician or radiologist without the need for additional support staff (26). Patient sedation is not usually required and the procedure is well tolerated, which allows for the establishment of a tissue diagnosis in patients who are too frail to undergo more invasive tests. Procedures are often carried out as day-cases and discharge is usually possible after a short period of observation.
Diagnostic yield
The sensitivity of image-guided biopsy has been reported in many observational series of malignant pleural disease. Both US- and CT-guided biopsies have been shown to have a clear advantage over blind pleural biopsy (21,30,32). Reported sensitivities range from 70% to 94%, and a number of studies have reported 100% specificity (21,28,29,33-36) (Table 1). There are a number of methods of increasing diagnostic yield. For example, a supra-diaphragmatic biopsy site and at least six biopsy samples have been shown to be of benefit (45,46). Unlike cytology, high yields are also found in the diagnosis of mesothelioma; current literature quotes US to have a yield of 77% and CT of 83.3% (30,35). Image-guided biopsy has an important role in suspected mesothelioma where there is no pleural effusion. Stigt et al. reported a diagnostic accuracy of 80% in a study of 14 patients who underwent US-guided biopsy with no or little pleural fluid (42).
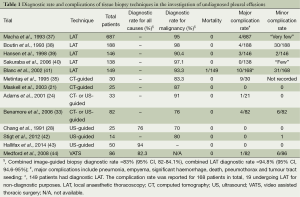
Full table
There are no randomised trials directly comparing the sensitivity of US- vs. CT-guided biopsy; however, published reports seem to show similar diagnostic yields between techniques (47). Sconfienza et al. published a retrospective comparison of outcomes in CT- and US-guided biopsies in 273 thoracic procedures (48). They included pleural (n=86) and lung parenchymal (n=187) biopsies, concluding that there was no difference in diagnostic sensitivity between the two methods and that US was significantly quicker and cheaper with less incidence of post-procedure pneumothorax.
Disadvantages
One of the disadvantages of CT-guided biopsy is the lack of real time ability to visualise the needle and tissue target. Fluoroscopy can overcome these issues, decreasing procedure time and reducing the number of passes of the biopsy needle (49). Fluoroscopy allows for biopsy of lesions not amenable to transbronchial or US-guided biopsy. One drawback is the radiation exposure to the radiologist who remains in the room throughout the procedure.
Whilst providing tissue to enable a diagnosis, image-guided biopsy does not provide any therapeutic benefit. It therefore follows that patients with large symptomatic effusions may be more appropriate for thoracoscopic drainage, biopsy and pleurodesis than an image-guided biopsy.
Whilst image-guided biopsy is more amenable in patients with a poor performance status, patients are required to breath-hold whilst image-guided biopsies are being taken. This may limit its use in patients with severe respiratory distress due to large pleural effusions or other underlying chest pathology (31).
Image-guided percutaneous biopsies are often angled which can lead to the distortion of anatomy, particularly in the case of mesothelial cells lying deep to the pleural surface. The presence of mesothelial cells within the extra pleural fat layer can indicate malignancy, and as such an ideal specimen will include this layer which can be difficult to achieve whilst also biopsying the abnormal pleura (33). Nevertheless, this is just one of many techniques used to distinguish malignant from benign histology; cytomorphology, cellularity, presence of necrosis and arrangement of cells is also useful. Advances in immunohistochemistry in recent years have proved incredibly useful at differentiating pleural malignancies and are now standard practice in all centres.
Complications
Image-guided biopsy is not without risk, although complication rates are low and vary between 0 and 10% (21,29). One study of 21 patients undergoing CT-guided biopsy for malignant mesothelioma reported one chest wall haematoma and one small haemoptysis, but neither required further intervention (29). Maskell et al. reported no complications in their image-guided arm of a randomised control trial comparing blind biopsy vs. CT-guided (21). Benamore et al. reported a low rate of new pneumothoraces detected on patient CT and chest radiographs, 11% and 4.7% respectively (33). In the same series of 85 patients undergoing CT- or US-guided biopsy, 7.5% were affected by bleeding at the time of biopsy, yet no patients went on to require a blood transfusion or chest drain insertion.
Video assisted thoracic surgery (VATS)
VATS is the gold standard for the diagnosis of pleural malignancy. However, it requires a general anaesthetic, a fully staffed operating theatre and an anaesthetist. VATS has a diagnostic yield of 90-95%, but due to its relative invasiveness is often only employed following negative closed biopsy and negative fluid cytology (50). VATS is rarely used as a purely diagnostic operation and patients often undergo further procedures during the operation. Patients undergoing VATS procedures often require different interventions to those undergoing LAT, such as extensive adhesiolysis, pleurectomy and decortications. Therefore, it is not surprising that complications are higher in VATS patients, with major complications being reported in up to 15% of cases (51). Elsewhere, major complications have been reported in just 1.2% of patients and no mortalities were recorded in an audit of one UK centre (44).
Local anaesthetic thoracoscopy (LAT)
Technique
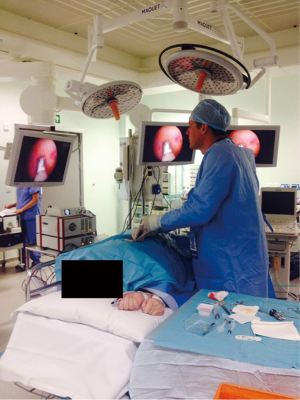
LAT involves the insertion of a port under local anaesthetic into the pleural space with the patient lying in the lateral decubitus position (Figure 1). A suction catheter is used to remove fluid, and a camera is introduced to visualise the pleura. Various instruments can then be used to biopsy areas of abnormal tissue and, if necessary, perform talc poudrage. The procedure is completed by inserting a chest drain, allowing for the re-expansion of the lung post-procedure (52). The majority of reported data concerning LAT has been with the use of a rigid scope. Nevertheless, it has been reported that a semi-flexible scope similar to those used in bronchoscopy can offer several advantages (53). There has not been widespread uptake of this technique as it limits the size of the pleural biopsies that can be obtained.
Advantages
One of the biggest advantages of LAT is that it can provide a combined diagnostic and therapeutic procedure in one setting. Thoracoscopy allows large volume thoracocentesis and direct visualisation of the pleura (Figure 2). Multiple biopsies can be taken through a single insertion point and, if necessary, talc poudrage can be performed to prevent recurrence of effusion (54). In some cases of undiagnosed pleural effusions, particularly in early pleural malignancy, radiological evidence of pleural thickening or nodules may be sparse. In such cases the lack of a target would challenge the use of image-guided biopsy; therefore direct examination of collapsed lung, diaphragm, visceral and parietal pleura and the outline of underlying ribs can identify appropriate areas for biopsy (52). Direct visualisation can discriminate between normal and abnormal areas, allowing for targeted biopsy of nodular, thickened or erythematous areas. In the absence of visual abnormality thoracoscopy allows for biopsies to be taken safely from the parietal pleura.
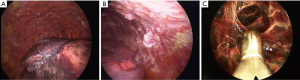
Thoracoscopy enables large volume fluid removal with a low risk of re-expansion pulmonary oedema, as pressure in the pleural space is immediately equalised by entry of air through the insertion port. de Campos et al. reported that 2.2% of patients undergoing thoracoscopy developed re-expansion pulmonary oedema (55). The risk of pulmonary oedema is increased by application of negative pressure or prolonged duration of collapse (56). If there is mediastinal shift present, then large volume fluid drainage is thought to be safe (4). Studies have reported the safe drainage of up to 8 L of pleural fluid without complications (57).
Malignant pleural effusions diagnosed via cytology, image-guided or blind biopsy may require a further pleural procedure to enable pleurodesis or insertion of an indwelling pleural catheter. Malignant pleural effusions treated by thoracentesis or chest drain insertion alone without pleurodesis, show a high recurrence rate and repeated procedures risk empyema and pneumothorax (9). There remains controversy over the optimal method of symptom control in malignant pleural effusions. Thoracoscopic talc poudrage, talc slurry pleurodesis and indwelling pleural catheters are all subject to current large clinical trials aiming to answer these uncertainties (58,59).
Diagnostic yield
A previously published selection of 22 case series assessing LAT for the diagnosis of malignant disease showed a 92.6% sensitivity (1,268/1,369; 95% CI, 91.1-94.0%) (60). In the case series some patients received both blind pleural biopsy and LAT. When those with positive blind biopsies were excluded, the sensitivity remained high at 90.1% (334/337; 95% CI, 86.6-92.9%). As well as in malignancy, LAT may be considered in patients with suspected TB where standard biopsy has failed to elicit a diagnosis. One study that directly compared LAT to Abrams needle biopsy found that thoracoscopy had 100% sensitivity for diagnosing TB (22). However, given that the same study found that blind pleural biopsy has a sensitivity of 79% in TB, this remains the investigation of choice in areas with a high burden of disease (22,23).
LAT has a low rate of complication and mortality despite being relatively invasive. Rahman et al. calculated the combined complications and mortality rates in 47 studies of LAT (60). They found a mortality rate of 0.34% (95% CI, 0.19-0.54), a large number of these (9/16) being from a large randomised control study of talc poudrage which led to the identification of the use of non-graded talc as a potentially harmful intervention (61). Major complications including empyema, haemorrhage, port site tumour growth, bronchopleural fistula, postoperative pneumothorax or air leak and pneumonia were reported in 1.8% of cases (95% CI, 1.4-2.2%).
Disadvantages
LAT is usually performed in an operating theatre or a procedure room with access to full hospital services if required. The procedure can take anywhere from 30 minutes to several hours depending on the technical difficulty and skill of the operator. Patients must be able to tolerate lying in the lateral decubitus position for the duration of the procedure. Therefore, those patients with a performance status of >2 are often unsuitable. However, many patients presenting with pleural effusions have fluid-related dyspnoea which can be rapidly controlled with large volume thoracocentesis during LAT. Uncontrollable coughing from pleural irritation or other causes can contraindicate the use of LAT as access and the ability to visualise the whole pleura becomes hazardous.
Contraindications
Several contraindications exist which can exclude a patient from being able to undergo LAT. If a lung has been unable to collapse due to obliteration of the pleural space by the underlying disease, thoracoscopy should not be attempted. Uncorrected bleeding disorders, cardiovascular instability, pulmonary hypertension and untreated hypoxaemia also prevent thoracoscopy (52). Severe obesity makes thoracoscopy technically difficult as port insertion may be limited due to depth of subcutaneous fat. Patients should also be medically optimised prior to the procedure including treatment of reversible conditions such as clotting dysfunction, renal failure or infection. Highly loculated fluid can also prevent thoracoscopy by preventing adequate lung collapse.
Identifying a safe site for port insertion whilst in the lateral decubitus position can be difficult in patients who have smaller effusions. In some instances an artificial pneumothorax must be created prior to port-insertion (62). This requires the introduction of air through a blunt needle under US guidance. The use of US prior to thoracoscopy has now become routine following several studies showing it can reduce the need for pneumothorax induction (63). In LAT, US visualisation of the pleural space can also reduce total procedure time and in one study prevented access failure in 100% of cases (37,63).
In-patient admission
The duration of hospital admission in patients with effusions, particularly with malignant aetiologies, is a key consideration in those with potentially short life expectancy. One prospective performance analysis of a thoracoscopy service in the UK reported the duration of patient stays following thoracoscopy and poudrage was 4.5 days (64). A combination of several other studies found a mean duration of 4.6 days (60). This data is relatively dated, and routine practice in the UK now dictates a single night’s admission following thoracoscopy. When compared to image-guided biopsy, where inpatient stay is often not required this may seem excessive; however, it must be considered that these patients may require further procedures to drain fluid and achieve adequate pleurodesis.
Tumour tract invasion
One of the biggest concerns in obtaining a tissue diagnosis in suspected pleural malignancy is the risk of tumour invasion of the tract formed by the biopsy instrument. Rates of tract seeding range from 0 to 40% (33,35,65) and can present as subcutaneous nodules of varying size. A systematic review of the literature found differences in rates of tumour seeding between biopsy techniques: thoracotomy showed the highest (24%), followed by thoracoscopy and image-guided biopsy at 9-16% and 0-22%, respectively (66). Prophylactic radiotherapy has been delivered post-procedure in many centres for the past two decades based on trials conducted in the 1990s (65,67). Its efficacy has been recently questioned following two small under-powered randomised controlled studies showing no benefit of radiotherapy to biopsy sites (68,69). The British Thoracic Society Statement on Mesothelioma in 2007 suggested there still may be a role for prophylactic radiotherapy in selected patients with good performance status (70). Currently there are two large randomised controlled trials looking to answer this question: the Prophylactic Irradiation of Tracts (PIT) and surgical and large bore pleural procedures in malignant pleural mesothelioma and radiotherapy trial (SMART) trials. These are due to report their findings within the next 12 months.
Training and competence aspects
All forms of biopsy techniques require adequate training and supervision to ensure safety and maximum diagnostic yield. However, image-guided techniques and thoracoscopy have different training demands. Respiratory and critical care trainees are expected to gain US proficiencies as part of their specialist training. Nevertheless, these proficiencies do not necessarily encompass pleural biopsy. In fact, level one competency recommended by the Royal College of Radiologists in the UK does not include tissue biopsy (71).
Internationally there are no agreed guidelines as to what constitutes adequate training to perform thoracoscopy. The American College of Chest Physicians set a minimum baseline of 20 supervised procedures before a trainee can be described as competent. A further ten procedures per year with adequate internal and external audit is required to maintain competence (72). The British Thoracic Society define three levels of competence in thoracoscopy, ranging from basic diagnostic and therapeutic procedures (level I) to VATS (level III) encompassing the competencies of a thoracic surgeon (60). The training required to perform thoracoscopy combined with the patient throughput required to maintain expertise means many hospitals are unable to offer thoracoscopy. Training in thoracoscopy is standard practice across Western Europe. However, in the U.S. a survey of trainee respiratory physicians found just 12% of training programmes included thoracoscopy (73,74). The uptake of thoracoscopy across the UK is increasing; in 5 years between 2004 and 2009 the number of centres offering thoracoscopy rose from just 17 to 37 (60). It has been predicted that demand for thoracoscopy may plateau as the rate of malignant mesothelioma reaches its peak, although it is likely to remain a powerful diagnostic and therapeutic tool (75).
Direct comparison
There are very few direct comparison trials between image-guided biopsy and thoracoscopy. Metintas et al. published a trial of 124 patients who were without a diagnosis following pleural fluid analysis and radiology (76). The patients were randomised to receive CT-guided Abrams needle biopsy (62 patients) or thoracoscopy (62 patients). CT-guided biopsy showed a diagnostic rate in malignancy or TB of 87.5% vs. 94.1% for thoracoscopy, a non-significant statistical difference (P=0.252). Complication rates were similar between both groups and only one major complication occurred in the CT-guided Abram’s needle biopsy group. The lack of direct comparison in published literature may indicate that both techniques occupy slightly different places within the diagnostic algorithms in pleural effusion.
Emerging themes in pleural biopsy
Physician led image-guided biopsy
Traditionally image-guided biopsies have been the domain of specialised radiologists. However, Diacon et al. reported an US-guided pleural biopsy service led by respiratory physicians (32). They were able to biopsy lesions of >20 mm under US guidance with a 14 gauge cutting needle, including suspected lung and pleural malignancies. In 91 patients they showed 85.5% sensitivity for malignancy and this was 100% for mesothelioma (10 patients). Two percent of patients required drainage for pneumothorax post-procedure. A recent retrospective review of practice in one UK pleural service analysed the use of physician based US-guided biopsy for pleural disease. Sufficient sample was obtained for histological diagnosis in 47/50 cases of physician based biopsy (43). In an interesting addition, the authors also introduced the use of US-guided biopsy in patients who had failed thoracoscopy. Patients were consented beforehand for both thoracoscopy and US-guided biopsy. Thirteen patients failed thoracoscopy and were converted on table to US biopsy and in these patients a definitive diagnosis was achieved in 85.6%. This novel approach can reduce the need for further admission and interventions and can provide a more timely diagnosis.
Day-case LAT
Hospital admission following thoracoscopy is standard practice worldwide. However, recently a group from the Mayo Clinic has reported a day-case procedure service (38,41,77,78). They were able to provide a LAT day service with an average time from pre-operative check-in to discharge of 294 minutes (range, 174 to 479 minutes). DePew et al. were able to achieve this by confirming lung re-expansion early in the post-procedural period and by using a standardised post-anaesthetic scoring system to assess eligibility for discharge (78). Of 51 patients treated by this method just three required overnight stay in hospital, two for persistent pain and one for post-procedural confusion. However, 27.5% of patients in this study required a further invasive pleural procedure indicating that day-case thoracoscopy is not suitable for all patients, particularly those requiring talc poudrage. It is yet to be seen whether day-case services will be established worldwide, despite their feasibility.
Conclusions: a considered approach
No single biopsy technique is appropriate for every patient presenting with pleural disease. Each technique has specific patient cohorts in which it will always be considered superior. Whilst direct comparison may be applicable in certain patient groups the vast majority of patients will have certain clinical and radiological characteristics which will lend themselves to a particular technique. The choice between different investigative procedures cannot be based solely on a comparison of diagnostic rates and complications, but also on factors such as control of pleural fluid production and patient preference. By considering these issues, individualised patient centered care can be delivered to a high standard.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Du Rand I, Maskell N. Introduction and methods: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii1-3. [PubMed]
- Sahn SA. Pleural effusions of extravascular origin. Clin Chest Med 2006;27:285-308. [PubMed]
- Hooper C, Lee YC, Maskell N, et al. Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii4-17. [PubMed]
- American Thoracic Society. Management of malignant pleural effusions. Am J Respir Crit Care Med 2000;162:1987-2001. [PubMed]
- Light RW. Clinical practice. Pleural effusion. N Engl J Med 2002;346:1971-7. [PubMed]
- Light RW, Macgregor MI, Luchsinger PC, et al. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972;77:507-13. [PubMed]
- Porcel JM, Esquerda A, Vives M, et al. Etiology of pleural effusions: analysis of more than 3,000 consecutive thoracenteses. Arch Bronconeumol 2014;50:161-5. [PubMed]
- Rodrîguez-Panadero F, Borderas Naranjo F, López Mejîas J. Pleural metastatic tumours and effusions. Frequency and pathogenic mechanisms in a post-mortem series. Eur Respir J 1989;2:366-9. [PubMed]
- Roberts ME, Neville E, Berrisford RG, et al. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii32-40. [PubMed]
- Hodgson JT, McElvenny DM, Darnton AJ, et al. The expected burden of mesothelioma mortality in Great Britain from 2002 to 2050. Br J Cancer 2005;92:587-93. [PubMed]
- Maskell NA, Butland RJ; Pleural Diseases Group, et al. BTS guidelines for the investigation of a unilateral pleural effusion in adults. Thorax 2003;58 Suppl 2:ii8-17. [PubMed]
- Abrams LD. A pleural-biopsy punch. Lancet 1958;1:30-1. [PubMed]
- Gordon S. Art. VIII.—Clinical reports of rare cases, occurring in the Whitworth and Hardwicke Hospitals. The Dublin Quarterly Journal of Medical Science 1866;41:83-99.
- Jacobaeus HC. Ueber die Möglichkeit die Zystoskopie die Untersuchung seroser Holungen anzuwenden. Münchener Medizinische Wochenschrift 1910;57:2090-2.
- vanSonnenberg E, Casola G, Ho M, et al. Difficult thoracic lesions: CT-guided biopsy experience in 150 cases. Radiology 1988;167:457-61. [PubMed]
- Donald I, Macvicar J, Brown TG. Investigation of abdominal masses by pulsed ultrasound. Lancet 1958;1:1188-95. [PubMed]
- Pell RL. Ultrasound for routine clinical investigations. Ultrasonics 1964;2:87-9.
- Renshaw AA, Dean BR, Antman KH, et al. The role of cytologic evaluation of pleural fluid in the diagnosis of malignant mesothelioma. Chest 1997;111:106-9. [PubMed]
- Whitaker D. The cytology of malignant mesothelioma. Cytopathology 2000;11:139-51. [PubMed]
- Clive AO, Kahan BC, Hooper CE, et al. Predicting survival in malignant pleural effusion: development and validation of the LENT prognostic score. Thorax 2014;69:1098-104. [PubMed]
- Maskell NA, Gleeson FV, Davies RJ. Standard pleural biopsy versus CT-guided cutting-needle biopsy for diagnosis of malignant disease in pleural effusions: a randomised controlled trial. Lancet 2003;361:1326-30. [PubMed]
- Diacon AH, Van de Wal BW, Wyser C, et al. Diagnostic tools in tuberculous pleurisy: a direct comparative study. Eur Respir J 2003;22:589-91. [PubMed]
- Loddenkemper R, Mai J, Scheffler N, et al. Prospective individual comparison of blind needle biopsy and of thoracoscopy in the diagnosis and differential diagnosis of tuberculous pleurisy. Scand J Respir Dis Suppl 1978;102:196-8. [PubMed]
- Adams RF, Gleeson FV. Percutaneous image-guided cutting-needle biopsy of the pleura in the presence of a suspected malignant effusion. Radiology 2001;219:510-4. [PubMed]
- Helm EJ, Gleeson FV. CT Guided Pleural Biopsy. Available online: http://www.toraks.org.tr/uploadFiles/book/file/242201117242-8791-CT-guided.pdf
- Koegelenberg CF, Diacon AH. Image-guided pleural biopsy. Curr Opin Pulm Med 2013;19:368-73. [PubMed]
- Koegelenberg CF, Diacon AH. Pleural controversy: close needle pleural biopsy or thoracoscopy-which first? Respirology 2011;16:738-46. [PubMed]
- Chang DB, Yang PC, Luh KT, et al. Ultrasound-guided pleural biopsy with Tru-Cut needle. Chest 1991;100:1328-33. [PubMed]
- Adams RF, Gray W, Davies RJ, et al. Percutaneous image-guided cutting needle biopsy of the pleura in the diagnosis of malignant mesothelioma. Chest 2001;120:1798-802. [PubMed]
- Heilo A, Stenwig AE, Solheim OP. Malignant pleural mesothelioma: US-guided histologic core-needle biopsy. Radiology 1999;211:657-9. [PubMed]
- Rahman NM, Gleeson FV. Image-guided pleural biopsy. Curr Opin Pulm Med 2008;14:331-6. [PubMed]
- Diacon AH, Schuurmans MM, Theron J, et al. Safety and yield of ultrasound-assisted transthoracic biopsy performed by pulmonologists. Respiration 2004;71:519-22. [PubMed]
- Benamore RE, Scott K, Richards CJ, et al. Image-guided pleural biopsy: diagnostic yield and complications. Clin Radiol 2006;61:700-5. [PubMed]
- Scott EM, Marshall TJ, Flower CD, et al. Diffuse pleural thickening: percutaneous CT-guided cutting needle biopsy. Radiology 1995;194:867-70. [PubMed]
- Metintaş M, Ozdemir N, Işiksoy S, et al. CT-guided pleural needle biopsy in the diagnosis of malignant mesothelioma. J Comput Assist Tomogr 1995;19:370-4. [PubMed]
- Mueller PR, Saini S, Simeone JF, et al. Image-guided pleural biopsies: indications, technique, and results in 23 patients. Radiology 1988;169:1-4. [PubMed]
- Macha HN, Reichle G, von Zwehl D, et al. The role of ultrasound assisted thoracoscopy in the diagnosis of pleural disease. Clinical experience in 687 cases. Eur J Cardiothorac Surg 1993;7:19-22. [PubMed]
- Boutin C, Rey F, Gouvernet J, et al. Thoracoscopy in pleural malignant mesothelioma: a prospective study of 188 consecutive patients. Part 2: Prognosis and staging. Cancer 1993;72:394-404. [PubMed]
- Hansen M, Faurschou P, Clementsen P. Medical thoracoscopy, results and complications in 146 patients: a retrospective study. Respir Med 1998;92:228-32. [PubMed]
- Sakuraba M, Masuda K, Hebisawa A, et al. Diagnostic value of thoracoscopic pleural biopsy for pleurisy under local anaesthesia. ANZ J Surg 2006;76:722-4. [PubMed]
- Blanc FX, Atassi K, Bignon J, et al. Diagnostic value of medical thoracoscopy in pleural disease: a 6-year retrospective study. Chest 2002;121:1677-83. [PubMed]
- Stigt JA, Boers JE, Groen HJ. Analysis of "dry" mesothelioma with ultrasound guided biopsies. Lung Cancer 2012;78:229-33. [PubMed]
- Hallifax RJ, Corcoran JP, Ahmed A, et al. Physician-based ultrasound-guided biopsy for diagnosing pleural disease. Chest 2014;146:1001-6. [PubMed]
- Medford AR, Awan YM, Marchbank A, et al. Diagnostic and therapeutic performance of video-assisted thoracoscopic surgery (VATS) in investigation and management of pleural exudates. Ann R Coll Surg Engl 2008;90:597-600. [PubMed]
- Kirsch CM, Kroe DM, Azzi RL, et al. The optimal number of pleural biopsy specimens for a diagnosis of tuberculous pleurisy. Chest 1997;112:702-6. [PubMed]
- Koegelenberg CF, Bolliger CT, Theron J, et al. Direct comparison of the diagnostic yield of ultrasound-assisted Abrams and Tru-Cut needle biopsies for pleural tuberculosis. Thorax 2010;65:857-62. [PubMed]
- Qureshi NR, Gleeson FV. Imaging of pleural disease. Clin Chest Med 2006;27:193-213. [PubMed]
- Sconfienza LM, Mauri G, Grossi F, et al. Pleural and peripheral lung lesions: comparison of US- and CT-guided biopsy. Radiology 2013;266:930-5. [PubMed]
- Heck SL, Blom P, Berstad A. Accuracy and complications in computed tomography fluoroscopy-guided needle biopsies of lung masses. Eur Radiol 2006;16:1387-92. [PubMed]
- Page RD, Jeffrey RR, Donnelly RJ. Thoracoscopy: a review of 121 consecutive surgical procedures. Ann Thorac Surg 1989;48:66-8. [PubMed]
- Harris RJ, Kavuru MS, Mehta AC, et al. The impact of thoracoscopy on the management of pleural disease. Chest 1995;107:845-52. [PubMed]
- Bhatnagar R, Maskell NA. Medical pleuroscopy. Clin Chest Med 2013;34:487-500. [PubMed]
- Lee P, Hsu A, Lo C, et al. Prospective evaluation of flex-rigid pleuroscopy for indeterminate pleural effusion: accuracy, safety and outcome. Respirology 2007;12:881-6. [PubMed]
- Canto A, Rivas J, Saumench J, et al. Points to consider when choosing a biopsy method in cases of pleurisy of unknown origin. Chest 1983;84:176-9. [PubMed]
- de Campos JR, Vargas FS, de Campos Werebe E, et al. Thoracoscopy talc poudrage: a 15-year experience. Chest 2001;119:801-6. [PubMed]
- Trapnell DH, Thurston JG. Unilateral pulmonary oedema after pleural aspiration. Lancet 1970;1:1367-9. [PubMed]
- Mohamed EE, Talaat IM, Abd Alla AE-DA, et al. Diagnosis of exudative pleural effusion using ultrasound guided versus medical thoracoscopic pleural biopsy. Egyptian Journal of Chest Diseases and Tuberculosis 2013;62:607-15.
- Lee P. Point: Should thoracoscopic talc pleurodesis be the first choice management for malignant effusion? Yes. Chest 2012;142:15-7; discussion 20-1.
- Light RW. Counterpoint: should thoracoscopic talc pleurodesis be the first choice management for malignant pleural effusion? No. Chest 2012;142:17-9; discussion 19-20.
- Rahman NM, Ali NJ, Brown G, et al. Local anaesthetic thoracoscopy: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii54-60. [PubMed]
- Dresler CM, Olak J, Herndon JE 2nd, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest 2005;127:909-15. [PubMed]
- Loddenkemper R. Thoracoscopy--state of the art. Eur Respir J 1998;11:213-21. [PubMed]
- Medford AR, Agrawal S, Bennett JA, et al. Thoracic ultrasound prior to medical thoracoscopy improves pleural access and predicts fibrous septation. Respirology 2010;15:804-8. [PubMed]
- Medford AR, Agrawal S, Free CM, et al. A local anaesthetic video-assisted thoracoscopy service: prospective performance analysis in a UK tertiary respiratory centre. Lung Cancer 2009;66:355-8. [PubMed]
- Boutin C, Rey F, Viallat JR. Prevention of malignant seeding after invasive diagnostic procedures in patients with pleural mesothelioma. A randomized trial of local radiotherapy. Chest 1995;108:754-8. [PubMed]
- Lee C, Bayman N, Swindell R, et al. Prophylactic radiotherapy to intervention sites in mesothelioma: a systematic review and survey of UK practice. Lung Cancer 2009;66:150-6. [PubMed]
- Low EM, Khoury GG, Matthews AW, et al. Prevention of tumour seeding following thoracoscopy in mesothelioma by prophylactic radiotherapy. Clin Oncol (R Coll Radiol) 1995;7:317-8. [PubMed]
- Bydder S, Phillips M, Joseph DJ, et al. A randomised trial of single-dose radiotherapy to prevent procedure tract metastasis by malignant mesothelioma. Br J Cancer 2004;91:9-10. [PubMed]
- O'Rourke N, Garcia JC, Paul J, et al. A randomised controlled trial of intervention site radiotherapy in malignant pleural mesothelioma. Radiother Oncol 2007;84:18-22. [PubMed]
- British Thoracic Society Standards of Care Committee. BTS statement on malignant mesothelioma in the UK, 2007. Thorax 2007;62 Suppl 2:ii1-ii19. [PubMed]
- The Royal College of Radiologists. eds. Ultrasound training recommendations for medical and surgical specialties, Second edition. London: The Royal College of Radiologists, 2012.
- Ernst A, Silvestri GA, Johnstone D, et al. Interventional pulmonary procedures: Guidelines from the American College of Chest Physicians. Chest 2003;123:1693-717. [PubMed]
- Pastis NJ, Nietert PJ, Silvestri GA, et al. Variation in training for interventional pulmonary procedures among US pulmonary/critical care fellowships: a survey of fellowship directors. Chest 2005;127:1614-21. [PubMed]
- Dijkman JH, Martinez Gonzales del Rio J, Loddenkemkper R, et al. Report of the working party of the "UEMS Monospeciality Section on Pneumology" on training requirements and facilities in Europe. Eur Respir J 1994;7:1019-22. [PubMed]
- Medford AR, Bennett JA, Free CM, et al. Current status of medical pleuroscopy. Clin Chest Med 2010;31:165-72. [PubMed]
- Metintas M, Ak G, Dundar E, et al. Medical thoracoscopy vs CT scan-guided Abrams pleural needle biopsy for diagnosis of patients with pleural effusions: a randomized, controlled trial. Chest 2010;137:1362-8. [PubMed]
- Colt HG. Thoracoscopy. A prospective study of safety and outcome. Chest 1995;108:324-9. [PubMed]
- DePew ZS, Wigle D, Mullon JJ, et al. Feasibility and safety of outpatient medical thoracoscopy at a large tertiary medical center: a collaborative medical-surgical initiative. Chest 2014;146:398-405. [PubMed]