Conventional transbronchial needle aspiration with 23 gauge needle: a preliminary study
Introduction
Data from the literature and clinical experience have conclusively demonstrated the superiority of endobronchial ultrasound (EBUS) guided transbronchial needle aspiration (TBNA) over conventional TBNA (cTBNA) in the diagnosis of hilar and mediastinal lymphadenopathy (1,2). However, the cost of the EBUS equipment, the different needle costs, the maintenance costs of EBUS, and the potential cost of acquiring operator skills might limit the application to most hospitals.
Nevertheless, considering that size and location of lymph nodes (LNs) are crucial predictive factors of a successful aspirate (3), it is reasonable to encourage the use of cTBNA when appropriate, as aptly said by Trisolini et al. “While waiting to buy a Ferrari, do not leave your current car in the garage!” (4). It is the case of large LNs (>1.5) in favourable locations (#4R and/or #7) in which cTBNA should be the first diagnostic step (5). cTBNA is a safe and minimally invasive procedure with a high yield for the diagnosis of large LNs in favourable locations (1,2); however, despite its proven efficacy and safety, it is usually underutilized by pulmonologists. Based on European and American data, the percentage of pulmonologists using cTBNA varies between 11% and 30% (6,7).
The risk of puncturing mediastinum vascular is one of the main limiting factor for cTBNA broad applicability; in this regards, although few cases of major bleeding complications have been reported (8-10), in daily practice the percentage of clinically relevant bleedings during cTBNA is very low.
In order to collect histological and cytological samples by cTBNA, a range of biopsy needles is available. The choice of gauge (G) needle should be based on targeted lesion’s characteristics (such as the suspected nature of the disease, LN size and location). The 20-22 G and the larger 19 G needles are usually used to obtain cytology and histology specimens, respectively; in particular, the use of 19 G needle is recommended in case of suspected lymphoma, sarcoidosis, or other granulomatous inflammation (11).
Recently, a 23 G needle (which costs 34, 37 €) has been commercialized, with the foreseen advantages of minimize bleeding’s risk and reduce costs.
The aim of our study was to analyze the sample adequacy, diagnostic accuracy and safety of cTBNA performed with the new 23 G needle in comparison with 21 and 22 G needles (average cost: 6,400 €).
Materials and methods
We retrospectively analysed records from patients who underwent bronchoscopy with cTBNA for LNs >1.5 cm in stations #4R and/or #7 at the Thoracic Endoscopy Unit of the University Hospital of Parma from January 1st, 2007 to October 31st, 2011.
cTBNA is the most operator dependent among all bronchoscopic techniques (12), not just for the technical aspects of the procedure, but for difficulties which are due to the proper identification of the area to be sampled. In this regard, in order to ameliorate this technique’s skill, some pulmonary anatomic knowledge are mandatory, such as the mediastinal anatomy, the lymphonode and carina displacement in relation to tracheobronchial tree during respiration (13) and the ability to merge radiologic information with endoscopic anatomy (14). In order to reduce all these technical and personal bias we analyzed only cases sampled by a single well-trained bronchoscopist, particularly skilful at cTBNA.
All bronchoscopic procedures were performed under local anesthesia with or withouth intravenous moderate sedation according to the patient-reported tolerance. After the initial inspection of the tracheobronchial tree, the most probable site of lymphadenopathy was selected and aspiration needle was inserted. Puncture was performed using either the jabbing technique, or the piggyback method. After successful puncture of the tracheobronchial wall, a negative suction was applied manually to the TBNA needle using a 50 mL syringe at the time of needle agitation within the LN, then the aspirated specimens was placed on glass slides.
Experienced cytobiologists performed an immediate rapide onside cytological evaluation (ROSE) of the aspirates in order to obtain maximal assurance that adequate sample was achieved. For ROSE the Diff-Quick staining method was used, permanent slides were routinely stained with the May-Grunwald Giemsa and Papanicolaou methods. The presence of malignant cells or granulomatous inflammation or, in the absence of malignancy or granulomas, the presence of a preponderance of lymphocytes defined the adequacy of the specimen. Inadequate samples contained a preponderance of bronchial cells, a minority or no lymphocytes, and no findings specific to a diagnosis. Suspicious cytologic results were categorized as adequate nondiagnostic samples.
If a sample was inadequate we repeated sampling up to a maximum of six passes in the same LN.
Bronchial and/or parenchymal lesion, when present, were sampled only if ROSE did not provide a definitive diagnosis.
The following variables were recorded for each patient: (I) age; (II) sex; (III) size of needle: specimens were obtained with No. 23 (Diener, Tuttlingen/Germany), No. 21 (NA-401D-1321, Olympus, Tokyo, Japan) or No. 22 (MW-122 BARD-Wang, Billerica, MA. USA) G needles; (I) sample adequacy; (II) diagnostic accuracy and (III) complications: defined as any symptoms requiring emergency evaluation, new or worsening thoracic pain, or haemoptysis.
The primary aim for the study was the comparison of different needles’ size in relationship to sample adequacy of the TBNA on a per-patient basis, defined as proportion of adequate sample/patient who underwent TBNA. The secondary aim was the comparison of different needles size based on to diagnostic accuracy of the TBNA on a per-patients basis, defined as proportion of diagnostic sample/patient who underwent TBNA. Furthermore, we investigated a possible relationship between different needles’ size and bronchoscopic complications.
The study was approved by the local Ethical Committee.
Statistical analysis
Data are reported as a percentage of the total, unless otherwise specified. Percentages were compared by with chi-squared test. A P value less than 0.05 was taken as significant.
Results
Five hundred patients underwent cTBNA from January 1st, 2007 to October 31st, 2011 (Figure 1).
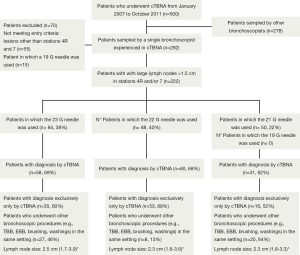
A total of 222 patients (186 men; mean age 63 years ±12, range 6-89) with LNs >1.5 cm in stations #4R and/or #7 were identified. A 23 G needle was used in 84 patients (38%), a 21 G needle in 88 patients (40%) and a 22 G needle in 50 patients (22%). Case distribution showed a dual allocation according to different needles’ availability: in the first period of the study the 21 or 22 G needles were used, in the second time the 23 G needle.
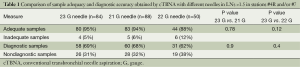
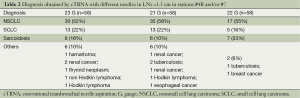
Tables 1 and 2 represent the comparison of sample adequacy and diagnostic accuracy obtained by cTBNA with different needles and diagnostic results, respectively.
Full table
Full table
No statistically significant differences between 23 G group and 21 or 22 G group in sample adequacy and diagnostic accuracy were found.
There were no intraprocedural or postprocedural complications related to needle’s size, and all bleeding complications were not clinically relevant.
Discussion
Our data showed that transbronchial 23 G needle is as safe and effective as 21 and 22 G needle to collect biological samples from large LNs in favourable mediastinal locations.
Only few studies have compared different types of cytologic transbronchial needles (15-17). Gittlen et al. (16) reported that a type IB (MW-122) Wang needle was superior to a type IIB (MW-222) cytology needle although the latter was more versatile. MW-122 is a needle with a larger lumen for the best suction ability and an inner steel stylet ending in a 13 mm 22 G needle. This needle can be used central lesions. MW-222 is a 22 G needle of the double lumen design, whereby the inner stylet may be partially withdrawan to allow more flexibility. This needle does not have as much suction capability as the type MW-122 but can be used both in peripheral and central lesions. Wang and Selcuk (15) showed that the number of positive aspirates obtained with W-220 needle was higher than those obtained with SW-221 (both from BARD-Wang, Billerica, MA, USA). In W-220 a 20 G double-lumen needle is attached to a spring allowing greater support and the development of momentum for increased puncture force. The retractable stylet within the inner lumen provides the proper combination of stiffness and flexibility. This needle can be used both in peripheral and central lesions. The SW-221 is a 21 G needle of a single-lumen design with a flexible inner guide to allow puncture of both central and peripheral areas. The inner stylet protrudes halfway into the lumen of the needle to “pack” cells. Spring allows for automatic readjust, no need manual stylet manipulation. Nakajima et al. (17) compared 21-G and 22-G needles during EBUS-TBNA reporting no differences in diagnostic yield.
In our case, although no significant differences were found in diagnostic accuracy or sample adequacy, the bronchoscopist noted that 23 G needle was easier to be used in angulated positions. Moreover, the thinner needle provided less bloody and contaminated specimens, as proven in previous study on thyroid glands (18) and pancreatic masses (19). Furthermore, 23 G needle used during cTBNA allows cost savings. In the current climate of restraint with respect to hospital costs, these financial factors must be considered in choosing a diagnostic test.
On the other hand, 23 G needle was more likely to bend and clog during the broncoscopic procedures. Moreover, on one occasion it happened that 23 G needle bent following contact with, although no medical complications were recorded. In this case, the needle and the bronchoscope were extracted as a unit from the trachea and passed carefully through the glottis and nose. The catheter was cut with metal scissors and withdrawn from the working channel without damaging the bronchoscope, nor airway damage. There have not been similar case reports with Wang or Olympus needles. Perforation of the plastic catheter was described with the MW-122 Wang needle but it was due to of an incorrect manipulation of the operator (20); similar to what we did, the needle and the bronchoscope were extracted as a unit from the trachea without airway damage.
We acknowledge the retrospective design as s potential limitation of this study, however the validity of the observation is supported by the followings: (I) although the bronchoscopic procedures were performed during four years and the operator experience is expected to improve over the time, our bronchoscopist had substantial experience of cTBNA even before the beginning of the revision; this is confirmed by the fact that the negative predictive value of our cTBNA samples remained consistent throughout the study period; (II) despite the fact that our study did not specifically examine the number of passes versus needle gauges, no significant difference in the mean number of passes between needle gauge were noted. In some patients, one pass of aspiration per target site was sufficient for ensure enough cellularity for diagnosis; in many other, multiple passes were performed; (III) in all cases a ROSE by an expert cytopathologists was perfomed, therefore results could not be generalized to all institutions with different expertise in this analysis. However, this limitation could be overcome in the light of a recent study (21) suggesting that a pulmonologist, after a short and intensive training, could be able to perform ROSE with good accuracy.
In the era of targeted therapy of lung cancer, every sampling technique must be able to provide adequate tissue for molecular evaluation that is necessary step for the treatment. In our study, we could not compare the adequacy of different needles in term of ability to perform somatic gene mutation analysis since precise typing in nonsmall cell carcinoma was not at that time required for therapeutic strategy. However, in a recent study (22), we showed that needle washing obtained in the course of cTBNA with 23 G needle allows reliable mutation testing (at least as regards EGFR and KRAS) and can be regarded as an additional important source of biological material for molecular profiling.
In summary, transbronchial 23 G needle is as safe and effective as the 21 and 22 G needle for the sampling of LNs >1.5 cm in stations #4R and/or #7. For this reason, to obtain cytology specimens from large LNs in favourable locations, the 23 G needle may represent an alternative and less expensive choice compared with 21 and 22 G needles, even if our observation needs to be confirmed in a larger prospective study.
Acknowledgements
The authors thank Professor Massimo Corradi for revision of the manuscript and the staff of bronchoscopy suite (Maria Teresa De Caprio, Rosalia Collura, Maria Grazia D’Auder, Maria Vittoria Di Maria, and Enrica Orlandi) for technical assistance. We also thank Dr. Sara Ramponi for her help in the statistical analysis.
Disclosure: The authors declare no conflict of interest.
References
- Jiang J, Browning R, Lechtzin N, et al. TBNA with and without EBUS: a comparative efficacy study for the diagnosis and staging of lung cancer. J Thorac Dis 2014;6:416-20. [PubMed]
- Gupta D, Dadhwal DS, Agarwal R, et al. Endobronchial ultrasound-guided transbronchial needle aspiration vs conventional transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest 2014;146:547-56. [PubMed]
- Bonifazi M, Zuccatosta L, Trisolini R, et al. Transbronchial needle aspiration: a systematic review on predictors of a successful aspirate. Respiration 2013;86:123-34. [PubMed]
- Trisolini R, Patelli M, Gasparini S. While waiting to buy a ferrari, do not leave your current car in the garage! Respiration 2010;79:452-3. [PubMed]
- Trisolini R, Gasparini S. Is it time for conventional TBNA to die? J Bronchology Interv Pulmonol 2013;20:368-9. [PubMed]
- Colt HG, Prakash UB, Offord KP. Bronchoscopy in North America: Survey by the American Association for Bronchology. J Bronchol 2000;7:8-25.
- Smyth CM, Stead RJ. Survey of flexible fibreoptic bronchoscopy in the United Kingdom. Eur Respir J 2002;19:458-63. [PubMed]
- Kucera RF, Wolfe GK, Perry ME. Hemomediastinum after transbronchial needle aspiration. Chest 1986;90:466. [PubMed]
- Lazzari Agli L, Trisolini R, Burzi M, et al. Mediastinal hematoma following transbronchial needle aspiration. Chest 2002;122:1106-7. [PubMed]
- Talebian M, Recanatini A, Zuccatosta L, et al. Hemomediastinum as a consequence of transbronchial needle aspiration. J Bronchol 2004;11:178-81.
- Wang KP, Metha A, Turner JF. Transbronchial needle aspiration for cytology and histology specimens. In: Wang KP, Metha A, Turner JF. eds. Flexible Bronchoscopy, ed 2. Cambridge: Blackwell Publishing, 2004:117-37.
- Holty JE, Kuschner WG, Gould MK. Accuracy of transbronchial needle aspiration for mediastinal staging of non-small cell lung cancer: a meta-analysis. Thorax 2005;60:949-55. [PubMed]
- Piet AH, Lagerwaard FJ, Kunst PW, et al. Can mediastinal nodal mobility explain the low yield rates for transbronchial needle aspiration without real-time imaging? Chest 2007;131:1783-7. [PubMed]
- Ceron L. “Tips and Pitfalls” in Transbronchial Needle Aspiration. J Bronchol 2005;12:245-49.
- Wang KP, Selcuk ZT. A comparative study of the Wang 21-gauge and 20-gauge needles for bronchoscopic use. J Bronchol 1997;4:201-4.
- Gittlen SD, Erozan Y, Wang KP. A new versatile transbronchial cytology needle for the staging and diagnosis of bronchogenic carcinoma. Chest 1988;94:561-5. [PubMed]
- Nakajima T, Yasufuku K, Takahashi R, et al. Comparison of 21-gauge and 22-gauge aspiration needle during endobronchial ultrasound-guided transbronchial needle aspiration. Respirology 2011;16:90-4. [PubMed]
- Degirmenci B, Haktanir A, Albayrak R, et al. Sonographically guided fine-needle biopsy of thyroid nodules: the effects of nodule characteristics, sampling technique, and needle size on the adequacy of cytological material. Clin Radiol 2007;62:798-803. [PubMed]
- Yusuf TE, Ho S, Pavey DA, et al. Retrospective analysis of the utility of endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) in pancreatic masses, using a 22-gauge or 25-gauge needle system: a multicenter experience. Endoscopy 2009;41:445-8. [PubMed]
- Fernández-Villar A, Leiro V, Blanco M, et al. Efficacy and safety of the eXcelon transbronchial aspiration needle in mediastinal lymph node enlargement: a case-control study. Respiration. 2007;74:208-13. [PubMed]
- Bonifazi M, Sediari M, Ferretti M, et al. The role of the pulmonologist in rapid on-site cytologic evaluation of transbronchial needle aspiration: a prospective study. Chest 2014;145:60-5. [PubMed]
- Bozzetti C, Naldi N, Nizzoli R, et al. Reliability of EGFR and KRAS mutation analysis on fine-needle aspiration washing in non-small cell lung cancer. Lung Cancer 2013;80:35-8. [PubMed]


