
Conversion ratio of tacrolimus switching from intravenous infusion to oral administration after lung transplantation
Introduction
Tacrolimus (FK506) belongs to the family of calcineurin inhibitors and is a first-line immunosuppressant to prevent rejection after organ transplantation. In the recent years, FK506 has been widely used to prevent graft-versus-host disease (GVHD) after organ transplantation (1-5). GVHD is one of the leading causes of death in patients after lung transplantation. Therefore, the prevention of severe GVHD is a crucial factor in the success of the treatment. The therapeutic window of tacrolimus is narrow, denoting that the therapeutic dose closes to the toxic dose. In addition, the pharmacokinetics shows a high inter-individual variability. The high blood concentration of tacrolimus might cause a variety of toxic and side effects; otherwise, graft rejection would occur owing to the insufficient blood concentration. Therefore, it is critical important to monitor the concentration of tacrolimus during treatment.
Fungal infections are prone to occur after lung transplantation. Therefore, the prophylactic use of antifungal drugs after lung transplantation commonly serves as a standard treatment option after lung transplantation (6,7). Caspofungin is one of the most widely used antifungal drugs in clinical practices and plays a key role in preventing and treating fungal infections after transplantation. However, as tacrolimus and caspofungin are used in combination, caspofungin would significantly inhibit the metabolism of tacrolimus and reduces the concentration of tacrolimus (8,9).
Tacrolimus is usually administrated through intravenous immediately after lung transplantation. Once the patient’s gastrointestinal tract tolerates the medicine (usually two to four days after surgery), oral administration would be used. Since the pharmacokinetics of intravenous infusion and oral administration of tacrolimus are markedly diverse with broadly range of bioavailability, it is difficult to determine the dose adjustment when there is a switch from continuous intravenous infusion to oral administration of tacrolimus, which leads to substantial change in its pharmacokinetic parameters and blood concentration (10). Thus, it would be of great importance to determine the conversion ratio of tacrolimus from intravenous to oral administration. However, the dose adjustment from intravenous to oral administration with drug combination of tacrolimus and caspofungin, has not been well elucidated in lung transplantation. In this study, we aimed to determine the conversion ratio of tacrolimus from intravenous to oral administration when used in combination with caspofungin after lung transplantation.
We present the following article in accordance with the “The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement” guideline checklist (available at http://dx.doi.org/10.21037/jtd-20-1191).
Methods
General information
Patients who underwent the first lung transplantation at the First Affiliated Hospital of Guangzhou Medical University from January 2015 to June 2019 were included. The inclusion criteria were as follow: (I) tacrolimus was first administered through intravenous infusion after lung transplantation and switched to oral treatment after the gastrointestinal function was restored; (II) caspofungin antifungal therapy was used; (III) tacrolimus blood concentration was monitored. The exclusion criteria: (I) patients with severe liver dysfunction: transaminase more than three times the normal value or Child-Pugh score >10 points; (II) patients taking fluconazole, voriconazole, or similar drugs that could significantly increase the blood concentration of tacrolimus; (III) cases where information was incomplete or could not be collected.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization. This study was approved by the First Affiliated Hospital of Guangzhou Medical University Medical Ethics Board (approval number 2020 No. K-08). All patients enrolled completed the informed consent form.
Study method
Immunosuppressive treatment plan
The triple immunosuppressive therapy with tacrolimus + Mofetil Mycophenolate + methylprednisolone was routinely performed after surgery. The initial intravenous tacrolimus dose was 0.02 mg/kg/day, with target blood concentration 10–15 ng/mL (11), the maintenance dose of the drug would be adjusted according to the blood concentration. As patients could tolerate oral administration of medicine (usually 2–4 days after surgery), the administration route of tacrolimus was switched from continuous intravenous infusion to daily oral capsules. The starting dose of oral administration of tacrolimus was 0.1 mg/kg/day, with target blood concentration 10–15 ng/mL, likewise, the maintenance dose of the drug would be adjusted according to the blood concentration. Once the oral administered of tacrolimus started, the intravenous administration route ceased.
Antifungal treatment plan
The dosage of caspofungin was 70 mg intravenous injection once on the first day after surgery and 50 mg intravenous injection once a day starting from the second day.
Determination of blood concentration of tacrolimus
The tacrolimus blood concentration was measured on the last day of intravenous administration and the second day of oral administration, with the whole blood trough concentration tested at 30 min before oral administration. A total of 2 mL of venous blood was drawn and placed in an anticoagulated blood collection tube with ethylenediaminetetraacetic acid (EDTA) for testing. The blood samples were sent to the Pharmacy Department of the First Affiliated Hospital of Guangzhou Medical University. The concentration of tacrolimus was monitored by the chemiluminescence microparticle immunoassay (CMIA) with Axsym System fluorescence polarization immunoassay (Abbott, USA). The calibration, quality control, and detection reagents were all from the AxSYM Vancomycin II Reagent Pack. The detection range was 0.5–1,000 ng/mL. The oral dosage of tacrolimus was adjusted by the clinician based on the patient’s conditions.
Data collection and evaluation
A retrospective study on patients after lung transplantation when tacrolimus was combined with caspofungin was used to compare the blood concentration/dose ratio [C/D, (ng/mL)/(mg/day)] of intravenously administered tacrolimus (C/Div), to the blood C/D for oral administration (C/Dpo). The conversion ratio of tacrolimus administration switched from the intravenous to the oral route was studied.
Statistical analysis
SPSS version 22.0 (SPSS Inc., Chicago, USA) and GraphPad Prism 5.0 (GraphPad Inc., USA) software was used to statistically analyze the data and compare the results from continuous intravenous administration to oral administration of tacrolimus. The blood concentration and C/D were compared using paired t test; the relationship between C/Div and C/Dpo was analyzed using linear regression. P<0.001 was considered statistically significant.
Results
Demographical information
Sixty-three patients were included in this study, including 39 males and 24 females, aged 33–75 years old. There were 25 cases of left lung transplantation, 14 cases of right lung transplantation, and 24 cases of double lung transplantation. The primary diseases were pulmonary interstitial fibrosis (29 cases), end-stage COPD (20 cases), bronchiectasis (9 cases), and silicosis (5 cases). The one-month survival rate was 88.9%, and the three-month survival rate was 75.2% (Table 1).

Full table
Blood concentration and C/D of tacrolimus switched from continuous intravenous administration to oral administration
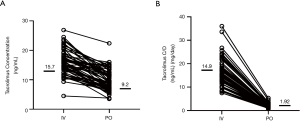
The median concentration of tacrolimus was 15.7 ng/mL (range, 4.5–26.9 ng/mL) during the continuous intravenous administration and 9.2 ng/mL (range, 3.5–22.4 ng/mL) after switching to oral administration (Figure 1A). The concentration of tacrolimus administered intravenously was significantly higher than that of oral administration (P<0.001). Similarly, the median value of C/Div was 14.9 [(ng/mL)/(mg/day)] {range, 7.5–36 [(ng/mL)/(mg/day)]}, and the median value of C/Div was 1.92 [(ng/mL)/(mg/day)] {range, 0.7–5.3 [(ng/mL)/(mg/day)]} (Figure 1B). The C/D of tacrolimus by intravenous administration was significantly higher than the oral C/D (P<0.001).
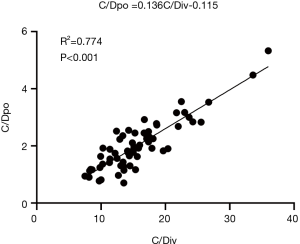
Correlation analysis between tacrolimus C/Dpo and C/Div
There was a significant correlation between tacrolimus C/Dpo and C/Div (R2 =0.774, P<0.001). The linear regression line in the figure indicated that the ratio of tacrolimus intravenous to oral conversion was 1:7.4 (Figure 2).
Discussion
Tacrolimus is a novel immunosuppressant with a 23-membered cyclic macrolide structure, which mainly inhibits T lymphocytes by locking the release of interleukin-2 (IL-2). It has the advantages of strong pharmacodynamics (10 to 100 times greater strength than that of cyclosporin A) (10), low dosage, high graft survival, and low incidence of acute rejection. The European Consensus Conference in 2009 has recommended its anti-rejection treatment for solid organ transplantation (12).
Lung transplantation patients are vulnerable to fungal infection, particularly of invasive aspergillosis (IPA), with a mortality rate of 74–92% (13). Although amphotericin B has been the first-line therapy in IPA, the severe side effects (e.g., nephrotoxicity, fever, chills) limit its widely use in clinical practice (14). Voriconazole has been approved as a effective drug against Aspergillu with generally good toleration and safety (15,16). However, voriconazole inhibits the cytochrome 450 and has severe drug interactions with calcineurin inhibitors such as cyclosporine and tacrolimus. As combined medication of tacrolimus and voriconazole are used, the concentration and immunosuppressive activity of tacrolimus increase (17), resulting in excessive immunosuppression. Caspofungin, has now become the first- or second-line therapy in lung transplant recipients for its remarkable efficacy and safety. The previous studies (18,19) reported that caspofungin acted as effective as amphotericin B in IPA, and had lower incidence of adverse events.
Since the gastrointestinal function of the patients after lung transplantation is not entirely restored, tacrolimus is commonly intravenously infused for 3–4 days, after that, the oral administration route will be sequentially used. However, the conversion ratio of tacrolimus from the intravenous to oral administration is difficult to determine due to the following reasons:
- The half-life (t1/2) of tacrolimus is 3.5 to 40.5 h, and the oral bioavailability ranges from 4% to 89% with an average of about 25% (20). If the same dose of tacrolimus was given orally as that administered intravenously, the drug blood concentration would be changed (10). The bioavailability results could not be used to estimate the conversion ratio of tacrolimus switching from intravenous administration to oral administration.
- Caspofungin could affect the blood concentration of tacrolimus. Tacrolimus is mainly metabolized by the liver cytochrome P450 (CYP450)-3A enzyme system and its blood concentration is highly related to the activity of metabolic enzymes (21). Caspofungin is mainly metabolized as an inactive substance in the liver, and serves as an inducer of liver drug-metabolizing enzymes, which can increase the metabolism of tacrolimus (22). Therefore, the dose of tacrolimus would be increased when used with caspofungin in combined therapy. The conventional oral dose of tacrolimus after lung transplantation is 0.08 mg/kg/day, whereas, in this study, the oral dose of tacrolimus was increased to 0.1 mg/kg/day (a 30% increase) due to the use of caspofungin.
- The therapeutic window of tacrolimus is narrow (23) (whole blood trough concentration needs to be maintained within 5–10 ng/mL), which denotes that the therapeutic dose is close to the toxic dose. The excessive blood concentration will cause kidney damage, and infection induced by excessive immunosuppression (24), whereas, an extremely low drug concentration may result in host rejection (25,26).
Therefore, in attempt to reduce the fluctuation of blood drug concentration, it would be great of importance to determine the conversion ratio of tacrolimus switching from intravenous to oral administration. Currently, the whole blood trough concentration is generally used as a monitoring index of tacrolimus, and the individual therapeutic dosage adjustments are accordingly performed in clinical practice. Studies have reported the optimal plasma concentration of tacrolimus ranged 10–15 ng/mL within ten months after lung transplantation (11). Therefore, it is necessary to routinely monitor the blood concentration of tacrolimus after lung transplantation, adjust the dosage according to the monitoring results to reach the target blood concentration to improve efficacy, and reduce the incidence of adverse reactions. Suetsugu et al. (27) showed that the conversion ratio of tacrolimus switched from intravenous to oral administration was 1:5 when combined medication with fluconazole, while the conversion ratio was 1:3 as tacrolimus used in combination with voriconazole or itraconazole. The reason for this difference is that tacrolimus is mainly metabolized by the liver CYP3A, while voriconazole is metabolized by CYP2C19, CYP2C9, and CYP3A4, and serves as a strong inhibitor of liver drug-metabolizing enzymes. Voriconazole’s inhibition of liver drug-metabolizing enzymes results in slower metabolism of tacrolimus, which leads to an increase in its blood concentration. However, to date, no dose adjustment of tacrolimus switching from the intravenous route to the oral route has been reported in patients with lung transplantation when the medicine was used in combination with caspofungin.
In this study, the regression analysis of the tacrolimus C/D for intravenous administration and oral administration showed that the slope of the regression line was 0.136, it was therefore plausible that the conversion ratio of tacrolimus switched from intravenous to oral administration was 1:7.4. Taken this into account, as patients with lung transplantation undergo combination therapy with caspofungin with the intravenous dose of tacrolimus is 1 mg/kg/d, the dose of 7.4 mg/kg/d is required when switching from the intravenous route to the oral route in order to maintain the blood drug concentration of tacrolimus. Tacrolimus is mainly metabolized by the liver, and the condition of liver function directly affects its blood concentration and the conversion ratio. Although liver function abnormalities are rare in patients after lung transplantation, this study excluded patients with liver dysfunction to obtain more reliable results by reducing the heterogeneity of results.
Some limitations should be acknowledged. Our study was conducted in a single center with small sample sizes, which limited the generalizability of our findings. The further prospective and long-term follow-up studies are needed to confirm the conversion ratio of tacrolimus from intravenous to oral administration when used in combination with caspofungin after lung transplantation. However, the current study provided a recommendation for the dose adjustment of tacrolimus for oral administration in patients undergoing lung transplantation, guiding the clinician to adjust the dosage and reduce the fluctuation of blood concentration of tacrolimus caused by the change of the administration route, all of which would considerably improve the clinical efficacy, avoid the adverse events, and provide a reference for the rational and safe use of tacrolimus in the clinical practice.
Conclusions
The conversion ratio of tacrolimus switched from intravenous administration to oral administration was 1:7.4 when tacrolimus was combined with caspofungin to treat patients with lung transplantation. Thus, when the intravenous dose of tacrolimus is 1 mg/kg/d, 7.4 mg/kg/d is required to switch to oral administration to maintain the same blood drug level.
Acknowledgments
Funding: Dr. Yang has received the National Key Research and Development Program of China (No.2018YFC1200100) and the Medical Research Foundation of Guangdong (No. A2020481). Dr. Zhang has received the National Natural Science Foundation of China [Grant Nos. 81700080 (RZ)], Dr. Li has received the National Natural Science Foundation of China (No. 81770079). Dr. Liu has received National Science and Technology Major Project (No. 2017ZX10204401003).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1191
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-20-1191
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1191
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1191). CY reports grants from National Key Research and Development Program of China, grants from Medical Research Foundation of Guangdong, grants from National Natural Science Foundation of China, grants from National Natural Science Foundation of China, grants from National Science and Technology Major Project, during the conduct of the study; RZ reports grants from National Natural Science Foundation of China, during the conduct of the study; YML reports grants from National Natural Science Foundation of China, during the conduct of the study; XQL reports grants from National Science and Technology Major Project, during the conduct of the study. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization. This study was reviewed and approved by the First Affiliated Hospital of Guangzhou Medical University Medical Ethics Board (approval number 2020 No. K-08). All patients enrolled completed the informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kur F, Reichenspurner H, Meiser BM, et al. Tacrolimus (FK506) as primary immunosuppressant after lung transplantation. Thorac Cardiovasc Surg 1999;47:174-8. [Crossref] [PubMed]
- Haddad EM, McAlister VC, Renouf E, et al. Cyclosporin versus tacrolimus for liver transplanted patients. Cochrane Database Syst Rev 2006.CD005161. [PubMed]
- Kaczmarek I, Zaruba MM, Beiras-Fernandez A, et al. Tacrolimus with mycophenolate mofetil or sirolimus compared with calcineurin inhibitor-free immunosuppression (sirolimus/mycophenolate mofetil) after heart transplantation: 5-year results. J Heart Lung Transplant 2013;32:277-84. [Crossref] [PubMed]
- Mayer AD, Dmitrewski J, Squifflet JP, et al. Multicenter randomized trial comparing tacrolimus (FK506) and cyclosporine in the prevention of renal allograft rejection: a report of the European Tacrolimus Multicenter Renal Study Group. Transplantation 1997;64:436-43. [Crossref] [PubMed]
- Penninga L, Penninga EI, Moller CH, et al. Tacrolimus versus cyclosporin as primary immunosuppression for lung transplant recipients. Cochrane Database Syst Rev 2013.CD008817. [PubMed]
- Bhaskaran A, Hosseini-Moghaddam SM, Rotstein C, et al. Mold infections in lung transplant recipients. Semin Respir Crit Care Med 2013;34:371-9. [Crossref] [PubMed]
- Burguete SR, Maselli DJ, Fernandez JF, et al. Lung transplant infection. Respirology 2013;18:22-38. [Crossref] [PubMed]
- Sable CA, Nguyen BY, Chodakewitz JA, et al. Safety and tolerability of caspofungin acetate in the treatment of fungal infections. Transpl Infect Dis 2002;4:25-30. [Crossref] [PubMed]
- Singh N, Limaye AP, Forrest G, et al. Combination of voriconazole and caspofungin as primary therapy for invasive aspergillosis in solid organ transplant recipients: a prospective, multicenter, observational study. Transplantation 2006;81:320-6. [Crossref] [PubMed]
- Kino T, Hatanaka H, Hashimoto M, et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. J Antibiot 1987;40:1249-55. [Crossref] [PubMed]
- Hirche TO, Knoop C, Hebestreit H, et al. Practical guidelines: lung transplantation in patients with cystic fibrosis. Pulm Med 2014;2014:621342.
- Wallemacq P, Armstrong VW, Brunet M, et al. Opportunities to optimize tacrolimus therapy in solid organ transplantation: report of the European consensus conference. Thera Drug Monitor 2009;31:139-52.
- Lin SJ, Schranz J, Teutsch SM. Aspergillosis case-fatality rate: systematic review of the literature. Clin Infect Dis 2001;32:358-66. [Crossref] [PubMed]
- Cannon JP, Garey KW, Danziger LH. A prospective and retrospective analysis of the nephrotoxicity and efficacy of lipid-based amphotericin B formulations. Pharmacotherapy 2001;21:1107-14. [Crossref] [PubMed]
- Dummer JS, Lazariashvilli N, Barnes J, et al. A survey of anti-fungal management in lung transplantation. J Heart Lung Transplant 2004;23:1376-81. [Crossref] [PubMed]
- Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 2002;347:408-15. [Crossref] [PubMed]
- Munoz P, Rodriguez C, Bouza E, et al. Risk factors of invasive aspergillosis after heart transplantation: protective role of oral itraconazole prophylaxis. Am J Transplant 2004;4:636-43. [Crossref] [PubMed]
- Kartsonis NA, Saah AJ, Joy Lipka C, et al. Salvage therapy with caspofungin for invasive aspergillosis: results from the caspofungin compassionate use study. J Infect 2005;50:196-205. [Crossref] [PubMed]
- Groetzner J, Kaczmarek I, Wittwer T, et al. Caspofungin as first-line therapy for the treatment of invasive aspergillosis after thoracic organ transplantation. J Heart Lung Transplant 2008;27:1-6. [Crossref] [PubMed]
- Venkataramanan R, Swaminathan A, Prasad T, et al. Clinical pharmacokinetics of tacrolimus. Clin Pharmacokinet 1995;29:404-30. [Crossref] [PubMed]
- Shiraga T, Matsuda H, Nagase K, et al. Metabolism of FK506, a potent immunosuppressive agent, by cytochrome P450 3A enzymes in rat, dog and human liver microsomes. Biochem Pharmacol 1994;47:727-35. [Crossref] [PubMed]
- Saner F, Gensicke J, Rath P, et al. Safety profile of concomitant use of caspofungin and cyclosporine or tacrolimus in liver transplant patients. Infection 2006;34:328-32. [Crossref] [PubMed]
- Chen YH, Zheng KL, Chen LZ, et al. Clinical pharmacokinetics of tacrolimus after the first oral administration in combination with mycophenolate mofetil and prednisone in Chinese renal transplant recipients. Transplant Proc 2005;37:4246-50. [Crossref] [PubMed]
- Kuypers DR, Claes K, Evenepoel P, et al. Clinical efficacy and toxicity profile of tacrolimus and mycophenolic acid in relation to combined long-term pharmacokinetics in de novo renal allograft recipients. Clin Pharmacol Ther 2004;75:434-47. [Crossref] [PubMed]
- Staatz C, Taylor P, Tett S. Low tacrolimus concentrations and increased risk of early acute rejection in adult renal transplantation. Nephrol Dial Transplant 2001;16:1905-9. [Crossref] [PubMed]
- Toda F, Tanabe K, Ito S, et al. Tacrolimus trough level adjustment after administration of fluconazole to kidney recipients. Transplant Proc 2002;34:1733-5. [Crossref] [PubMed]
- Suetsugu K, Ikesue H, Miyamoto T, et al. Analysis of the variable factors influencing tacrolimus blood concentration during the switch from continuous intravenous infusion to oral administration after allogeneic hematopoietic stem cell transplantation. Int J Hematol 2017;105:361-8. [Crossref] [PubMed]


