|
Review Article
Revisiting signs, strengths and weaknesses of Standard Chest
Radiography in patients of Acute Dyspnea in the Emergency
Department
Luciano Cardinale1, Giovanni Volpicelli2, Alessandro Lamorte2, Jessica Martino1, Andrea veltri1
1Istitute of Radiology, San Luigi Gonzaga Hospital, 10043 Orbassano (TO), Italy; 2Department of Emergency Medicine, San Luigi Gonzaga Hospital, 10043 Orbassano (TO), Italy
Corresponding to: Dr. Luciano Cardinale. Istitute of Radiology, San Luigi Gonzaga
Hospital, 10043 Orbassano (TO), Italy. Email: luciano.cardinale@gmail.com; Dr. Jessica Martino. Istitute of Radiology, San Luigi Gonzaga Hospital, 10043 Orbassano (TO), Italy. Email: jessica.martino85@gmail.com.
|
|
Abstract
Dyspnoea, defined as an uncomfortable awareness of breathing, together with thoracic pain are two of the most frequent
symptoms of presentation of thoracic diseases in the Emergency Department (ED). Causes of dyspnoea are various and
involve not only cardiovascular and respiratory systems. In the emergency setting, thoracic imaging by standard chest
X-ray (CXR) plays a crucial role in the diagnostic process, because it is of fast execution and relatively not expensive.
Although radiologists are responsible for the final reading of chest radiographs, very often the clinicians, and in particular
the emergency physicians, are alone in the emergency room facing this task. In literature many studies have demonstrated
how important and essential is an accurate direct interpretation by the clinician without the need of an immediate reading
by the radiologist. Moreover, the sensitivity of CXR is much impaired when the study is performed at bedside by portable
machines, particularly in the diagnosis of some important causes of acute dyspnoea, such as pulmonary embolism,
pneumothorax, and pulmonary edema. In these cases, a high inter-observer variability of bedside CXR reading limits the
diagnostic usefulness of the methodology and complicates the differential diagnosis. The aim of this review is to analyze the
radiologic signs and the correct use of CXR in the most important conditions that cause cardiac and pulmonary dyspnoea,
as acute exacerbation of chronic obstructive pulmonary disease, acute pulmonary oedema, acute pulmonary tromboembolism,
pneumothorax and pleural effusion, and to focus indications and limitations of this diagnostic tool.
Key words
Dyspnoea; chest X-ray; pulmonary oedema; heart failure; pleural effusion
J Thorac Dis 2012;4(4):398-407. DOI: 10.3978/j.issn.2072-1439.2012.05.05 |
|
Introduction
Dyspnoea and thoracic pain are the most frequent symptoms of
presentation of thoracic diseases in the Emergency Department
(ED). In the emergency setting, thoracic imaging and, first of all,
standard chest X-ray (CXR) play a crucial role in the diagnostic
process. According to one prospective observational study, the
most common diagnoses among elderly patients presenting to an ED with a complaint of acute shortness of breath or dyspnoea
are decompensated heart failure, pneumonia, chronic obstructive
pulmonary disease, pulmonary embolism, and asthma ( 1). A CXR is frequently helpful in evaluating patients with
dyspnoea. Characteristic roentgenographic findings occur in
patients with congestive heart failure and pneumonia, and
pulmonary fibrosis. The chest radiograph may also be abnormal
in patients with obstructive pulmonary disease, but the chest
film (particularly the bedside chest film) have low sensitivity
above all for the detection of airflow obstruction or pulmonary
embolism ( 2). Dyspnoea is defined as an uncomfortable awareness of
breathing. NYHA classified dyspnoea in four classes, according
to the functional decrease performance status of patients: in
the I class dyspnoea appears after moderate physical effort, in
the II class dyspnoea appears during normal activities, in the III
class dyspnoea appears for lower physical efforts, in the IV class
dyspnoea is always present ( 3). Causes of dyspnoea are various
and can involve mainly cardiovascular and respiratory apparatus. The aim of this script is to analyze the correct use of CXR in
the most important conditions causing cardiac and pulmonary
dyspnoea, and to focus indications and limitations of this
diagnostic tool. |
|
Acute exacerbation of chronic obstructive pulmonary disease
Chronic obstructive pulmonary disease (COPD) is a syndrome
characterized by a progressive limitation to the air flow, poorly
reversible and associated with an inflammatory response of
airway epithelium. Within this definition we can find both
chronic bronchitis and emphysema. Pathophysiological tests
can demonstrate a persistent reduction of FEV1 and FEV1/
FVC. Presentation of COPD is characterized by persistent
exertional dyspnoea, that can worsen during infective
exacerbations. During exacerbations it is possible to observe
hypoxemia and hypercapnia, while the sputum become
abundant and purulent.
Patients with COPD usually have one or two exacerbations
per year, often needing hospitalization, with an overall mortality
of 3-4%. Incidence of death is higher in the intensive care unit
(24%) ( 4). Most exacerbations are due to infections of the upper
airways ( 4). In the most severe cases, it is common to observe
co-morbidity with congestive heart failure, extra-pulmonary
infections or pulmonary embolism. Role, principal aspects and limitations of chest X-ray
In patients with COPD, diagnosis of exacerbation is possible
by evaluating clinical history, symptoms and physical signs,
even if instrumental examination is crucial for confirmation and
assessment of the severity. Very often COPD exacerbation with
involvement of large and/or small airways is not associated with
radiographic signs. CXR demonstrates abnormal images only in
16% of cases, mainly limited to signs of inflammatory infiltrates
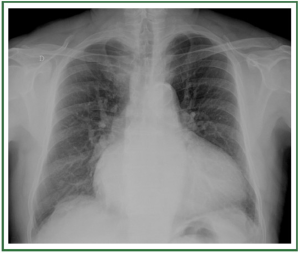
or pulmonary congestion ( 5-7) ( Figure 1). For these reasons CXR is not recommended as a routine
exam, but only in cases of suspected pneumonia, or to ruleout
other causes of dyspnoea, such as massive pleural effusion,
atelectasis, pneumothorax, pulmonary edema.
Other limitations of CXR in the diagnostic procedure
of exacerbation of COPD are high inter- and intraobserver
variability, but also low rates of agreement among radiologists
regarding the interpretation of pneumonia signs. Rates of
agreement for the diagnosis of pneumonia are even lower among
trainees or non-radiologist practitioners ( 8, 9). |
|
Acute pulmonary oedema
Acute pulmonary oedema (APE) is a condition of increased
fluid content of the lung, at the expense of its content of air.
It is classified into two main groups, depending on different
mechanisms: Cardiogenic APE, due to increased hydrostatic
pressure in pulmonary capillaries during congestive heart
failure or fluids excess; non cardiogenic or lesional APE, due to
increased capillary permeability during acute respiratory distress
syndrome (ARDS).
Differential diagnosis between cardiogenic and lesional
oedema often is not easy, even if the history recording with
description of symptoms, clinical findings at examination, time
course during hospital stay and treatment response, are all of
great help. Nevertheless, a correct differential diagnosis cannot
always be clarified, particularly in the critically ill patients.
Role, Main findings and limitations of standardard chest X-ray
CXR represents the first line imaging exam in a patients presenting
to the ED complaining of acute dyspnoea. The possibility of
correct diagnosis at CXR is directly proportional to the severity
and the duration of pulmonary congestion. The role of CXR is
not only the first diagnosis of APE, but also the differentiation
between cardiogenic and non-cardiogenic causes ( 10) and guiding
treatment. To these purposes, the radiologic signs and findings to be studied
are: the perfusion pattern and the spatial distribution of oedema, the
size of the vascular peduncle and the cardiac volume. Moreover, it
is highly important the recognition of some specific signs, like lung interstitial oedema, pleural effusion and air bronchogram.
In cardiogenic pulmonary edema, CXR may show cardiomegaly,
pulmonary venous hypertension, and pleural effusions. Radiologic
signs of cardiogenic APE are related to the severity of the condition,
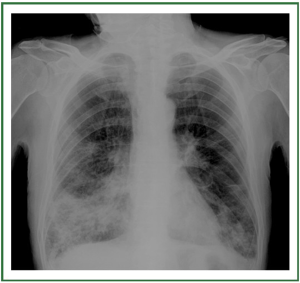
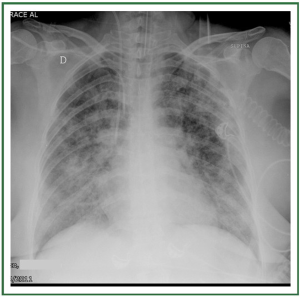
and may be divided into 3 stages ( Table 1) ( 11, 12). In stage I, an
upright examination demonstrates redistribution of blood flow
to the nondependent portions of the lungs and the upper lobes
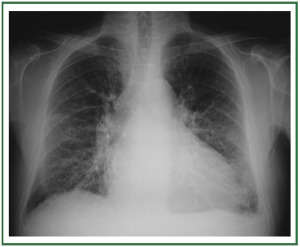
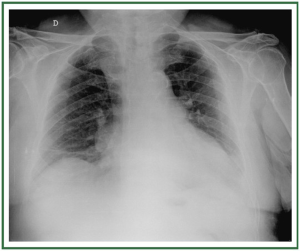
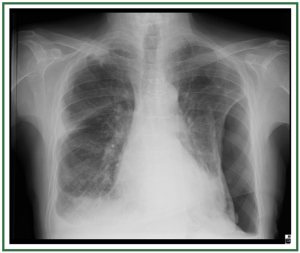
( Figure 2). In stage II, there is evidence of interstitial edema
with ill-defined vessels and peribronchial cuffing, as well as
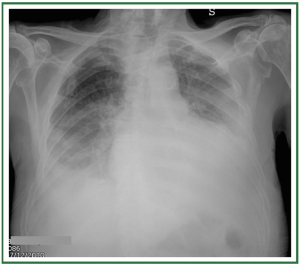
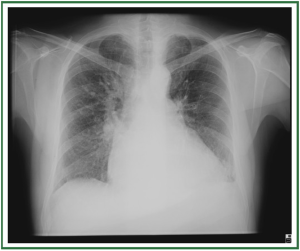
interlobular septal thickening ( Figure 3). In stage III, perihilar
and lower-lobe airspace filling is evident, with features typical
of consolidation (e.g., confluent opacities, and the inability to
see pulmonary vessels in the area of abnormality) ( Figure 4).
The airspace edema tends to spare the periphery in the mid
and upper lung. The distribution of the alveolar edema can be
influenced by:
• Gravity: supine or erect position and right or left decubitus
position;
• Obstructive lung disease, i.e. fluid leakage into the less
severe diseased areas of the lung.
| Table 1. Stage of congestive heart faliure. PCWP = pulmonary
capillary wedge pressure. |
Stage 1
Redistribution
PCWP 13-18 mmHg |
-Redistribution of pulmonary vessels
-Cardiomegaly
-Broad Vascular Pedicle (non acute CHF) |
Stage 2
Interstitial Edema
PCWP18-25 mmHg |
-Kerley lines
-Perbronchial cuffing
-Hazy contour of vessels
-Subpleural edema |
Stage 3
Alveolar Edema
PCWP > 25 mmHg |
-Consolidation
-Butterfly appearance
-Cottonwool appearance
-Pleural effusion |
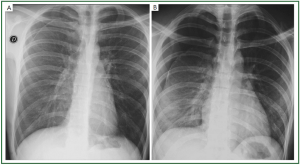
In non-cardiogenic causes, cardiomegaly and pleural effusions
are usually absent. The edema may be interstitial but is more
often consolidative. The cephalization of blood flow is missing,
though there may be shift of blood flow to less affected areas. The
edema is diffuse and does not spare the periphery of the mid or
upper lungs ( Table 2) ( Figure 5).
| Table 2. Radiographics features of pulmonary edema. Modified from Milne et al. |
| |
Cardiac |
ARDS |
|---|
| Heart size |
Enlarged |
Normal |
| Vascular pedicle |
Normal or enlarged |
Normal or reduced |
| Pulmonary blood flow distribution |
Inverted |
Balanced |
| Perbronchial cuffs |
Common |
Not common |
| Regional distribution lung edema |
Evcn |
Peripheral/patchy |
| Ari bronchogram |
Not common |
Very common |
| Plcural effusion |
Very common |
Not common |
In cases of large, acute myocardial infarction (MI) and
infarction of the mitral valve, support apparatus may produce
atypical patterns of pulmonary edema that may mimic
noncardiogenic edema or in some cases even a pneumonia.
CXR is moderately specific (specificity 76%, 83%), but not
very sensitive (67-68%) for the diagnosis of heart failure ( 13).
Therefore, CXR does not have a direct role in the pathway for the
positive diagnosis of heart failure, where the key investigation is
echocardiography. The main reason of this limitation is that CXR
is not sensitive enough to rule out heart failure in the presence
of a normal radiologic pattern or specific enough to rule - in the
condition in the presence of an abnormal pattern. However,
CXR is helpful,to rule-out other conditionsa that may enter the
differential diagnosis. |
|
Acute pulmonary trombo-embolism
Acute pulmonary thrombo-embolism (APT) is secondary to
sudden interruption or significant reduction of blood supply to
the lung due to pulmonary circulation obstruction, in most cases
due to embolization of thrombi originated from deep veins,
right cardiac chambers or, rarely, from the same pulmonary
circulation.
This pathologic condition is quite frequent and sometimes
constitutes a hemodynamic and respiratory emergency,
leading to death in 30% of untreated cases ( 14, 15). To date,
APT is considered the third leading cause of death in western
countries and the most misdiagnosed pathologic condition,
being correctly diagnosed only in 20% of cases ( 16). Physical
signs as well as routine diagnostic tests are not enough accurate
for a safe diagnosis of the condition. Indeed, history, physical
examination and blood d-dimer are useful to hypothesize APT
in the emergency setting and determine the pre-test probability
according to the criteria published by Wells and co-authors ( 17). Hemodynamic and clinic consequences of APT are directly
related to the extension and stability of the occlusion, as well
as the number of obstructed vessels serving the eighteen
identifiable bilateral lung segments. From the anatomic point
of view, it is common to differentiate three degrees of severity:
slight (reduction inferior to 40% of flow), severe (40-60%
of flow obstruction) and massive (over 60% obstruction).
This classification does not necessarily coincide with the
clinical definition of massive APT, that relies exclusively on
hemodynamic criteria. When vascular embolic obstruction is
superior to 80%, electromechanic dissociation and sudden death
usually follow ( 16). Obviously, hemodynamic and respiratory
consequences are widely variable, depending on co-morbidities
and pre-existing health status. Clinics of APT may widely vary from complete lack of symptoms, usually in small segmental
or sub-segmental embolism, to severe manifestations as acute
respiratory failure, hemodynamic shock and cardiac arrest ( 18). Role, main findings and limitations of standardard chest X-ray
CXR has a limited role in the diagnostic process of APT,
primarily related to the exclusion of other common causes of
respiratory failure and chest pain, because it is burdened by a low
sensivity and specificity.
Quite often, CXR is completely normal in APT. Instead,
spiral angio-CT (SCT) scan has a well defined role and it is the
first level radiographic test when a clinical suspicion has been
hypothesized and classified by the clinician ( 19, 20). SCT has a
higher sensitivity (87% vs. 33%) and specificity (95% vs. 59%)
over CXR, and indubitable advantages due to its fast execution,
broad view and objective interpretation, as well as its ability to
allow for other diagnoses when the initial clinical suspicion is
excluded ( 21). Limitations of CXR are related to the difficulty to recognize
specific signs. Some radiologic findings have been corroborated
in many years of experience. They have been argued by the careful
observation of CXR studies in patients with confirmed APT, but
rarely such signs are found altogether even in case of clear clinic
presentations ( 22). Nevertheless, many authors suggest that a
careful observation of CXR images can show some non specific
abnormalities in at least 90% of the cases ( 23-25). The possible
findings of standard CXR in APT are the following ( 16, 26):
(I) Pulmonary infiltrates, due to haemorrhagic or
oedematous infiltration of secondary lobules, often multiple and
presenting as round foci of alveolar consolidations or irregular
jeopardized opacities, without a segmental arrangement, more
often located to the right base, sometimes associated with signs
of atelectasis or pleural effusion.
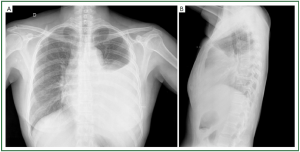
(II) Atelectasis, often sub-segmental, appearing as curved
lines reaching the pleura, secondary to alveolar collapse (line
of Fleishner), caused by bronchial obstruction due to mucosa
congestion, or alveolar collapse secondary to surfactant
reduction, or hypoventilation due to reduced diaphragmatic
excursion ( Figure 6).
(III) Diaphragm elevation secondary not only to reduction
of pulmonary volume due to the reduction in surfactant,
but mainly to the dysventilation due to reduced respiration
movement during pleural pain ( Figure 6).
(IV) Pleural effusion, mainly serous, bilateral and of slight
entity, often in association with basal atelectasis.
(V) Westermark sign, uncommon but highly specific,
corresponds to a region of impaired vascularisation in the lung
region distally to the site of the embolism ( Figure 7) ( 27).
Sometimes it is associated with deletion and dilation of the
affected pulmonary branch (more often the right pulmonary
artery). For a safe interpretation of this sign when present, the
film should be compared with an old radiogram where it was
absent. Another limitation of this sign is linked to the difficult
visualization when CXR is performed in the supine patient.
(VI) Right heart and azygos vein enlargement are signs
of severe pulmonary hypertension and right heart failure. They
are invariably associated with symmetric enlargement of the
ilar regions and other signs previously described. As for the
Westermark sign, visualization of these signs should always be compared with previous images and they are unreliable when
examination is performed in the recumbent position.
(VII) Hampton’s hump is a triangular opacity with the apex
pointing to the hilar region, sometimes with blurred margins and
irregular shape. It is a sign of interruption of blood supply from
the systemic circulation in the lung region previously excluded
by embolic obstruction of the functional circulation. It is more
frequent when APT overlaps with some pre-existing conditions,
like venous pulmonary hypertension, cardiac illnesses with left
heart failure and COPD. Very often this sign is associated to
pleural effusion. Often, the differential diagnosis with an alveolar
consolidation due to pneumonia is difficult. Despite the numerous signs listed, the most useful and
accurate radiologic finding is the normal appearance of CXR in
the face of patients presenting with acute dyspnoea or thoracic
pain. This observation has the value of excluding from the
differential other conditions potentially causing acute respiratory
failure and chest pain ( 16). |
|
Pneumothorax
Pneumothorax is defined as the presence of air in the pleural
cavity, with secondary lung collapse ( 28). It is usually classified
into spontaneous, when it occurs without a preceding event;
traumatic, due to direct or indirect trauma; and iatrogenic,
categorized by some investigators as a subdivision of traumatic
pneumothorax ( 29). Spontaneous pneumothorax is the largest
group and is classified into primary spontaneous pneumothorax
(PSP) and secondary spontaneous pneumothorax (SSP). PSP
occurs in young patients without obvious underlying lung
disease, and is usually caused by the rupture of a sub-pleural bleb.
SSP occurs as a complication of an underlying lung disease, most
often COPD or pulmonary tuberculosis ( 29, 30). Pneumothorax can be complete, with totally collapsed lung,
or small with little or no consequences. Continuous introduction
of air after every breath without possibility of release, because of
a valve mechanism, determines a life-threatening situation that
is indicated as tension pneumothorax. Clinical consequences
of pneumothorax are strictly connected with the timing of
interventions and pre-existing condition of the patient.
Role, principal aspects and limits of chest X-ray
Standard CXR, acquired in orthostatic position, is the elective
exam for the diagnosis. Signs used are better visible by
acquisition of a forced-expiration imaging ( Figure 8). When air
is collected between the two pleura layers, the visceral pleura
becomes visible as a thin diaphanous line, with no broncovascular
texture beyond it. Although highly specific, the
detection of this sign has a low sensitivity particularly when
CXR is performed in the supine position at bedside. A large
number of pneumothoraces (probably more than 30%) are not diagnosed by conventional CXR, particularly when expiration and
orthostatic radiograms cannot be obtained for clinical reasons ( 31).
When an anterior/posterior view obtained from a supine patient
is evaluated, diagnosis is more difficult because there is the
possibility to misdiagnose even large pneumothoraces, because
air move up and medially between the lung and the heart. Only
after having filled these spaces, free air can gather the usual
apical-lateral position ( 4). When a CXR is not acquired in an orthostatic posterioranterior
view, there are some other indirect signs that can
be important for diagnosing pneumothorax. These are the
emphasized radiolucency of the paracardiac region, the deep
sulcus sign ( 32), the appearance of sharp edges of mediastinum,
heart and subcutaneous tissues, or the visibility of the anteriorinferior
edge of the lung ( 33). Anyway, these signs are
pathognomonic but not constant. When possible, in doubtful cases acquisition of a radiogram in
the lateral view (Hessen position) or during a forced expiration,
can be useful ( 21, 34). In these cases, it is sometimes possible to
demonstrate even the smaller layer of pneumothorax. Free air can also collect in a fissure or behind the triangular
ligament, or it can distribute around an atelectasis or a
consolidated lobe, sometimes with unusual aspects against the
expected gravity distribution. This is due to variations of intrapleural
pressure in presence of various chronic pulmonary
diseases ( Figure 9). In these cases the differential diagnosis between pneumothorax,
pneumo-pericardium and pneumo-mediastinum at CXR can be
very difficult.
Diagnosis of tension pneumothorax is generally based mainly
on the first clinical evaluation because it gives usually clear
physical signs that may evolve rapidly to hemodynamic shock
and cardiac arrest. When the clinical conditions are not rapidly
evolving, CXR may be helpful in the early diagnosis allowing the
emergency physician a greater confidence in deciding aggressive
life-saving decompression treatment. The main radiologic
signs of tension pneumothorax are the lateral shift of heart and
mediastinum, the lowering of the hemi-diaphragm, the flattening
of the cardiac profile, the reduced size of the superior vena cava
and the protrusion of the parietal pleural layer between the
intercostal spaces.
The underused thoracic sonography has been widely
showed to be of great usefulness in the emergency diagnosis
of pneumothorax and even in the detection of radio-occult
pneumothorax, being far more accurate than CXR and
equivalent to CT scan ( 35). Its advantages include the fact that
it can easily and quickly be performed at the bedside by a wide
range of operators, such as trauma, emergency, and critical care
physicians ( 36). |
|
Pleural effusion
Pleural effusion is defined as the presence of fluid in excess
inside the pleural cavity. A thin fluid film is regularly present
between the two pleural layers, thus facilitating respiratory
sliding. A minimal amount of pleural fluid can be detected in
10% of healthy subjects, and it is physiologically increased after
laparotomy or in post-partum ( 37-39). Several conditions can cause pleural ef f usion, as
cardiovascular diseases, hyper-expansion of body fluids due
to renal and hepatic failure, infections, autoimmune disorders,
cancer and traumas ( 40). Role, principal aspects and limitations of chest X-ray
CXR is always been considered the first line diagnostic tool to
be used in the diagnosis and quantification of pleural effusion.
Orthostatic standard CXR in two views is able to detect even
a minimum amount of pleural effusion (about 25 mL), which
are usually visualized at lateral view only in the posterior
costophrenic angle. When some fluid is visualized also in the
lateral costophrenic angle at the posterior-anterior view, it is
possible to calculate a total amount of about 100 ml. Anyway,
severity of the disorder, lung and chest wall compliance,
capillarity of the pleural layers and the physical features of the
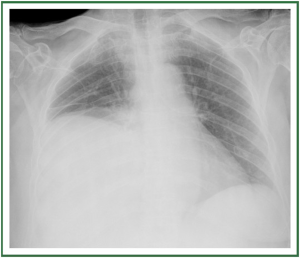
fluid, influence the spatial distribution in the pleural cavity ( 41). Classical radiologic signs are consistent with a dependent
opacity with lateral upward sloping of a meniscus-shaped
contour. The diaphragmatic contour is partially or completely
obliterated, depending on the amount of collected fluid
(silhouette sign) ( Figure 10A, B). In case of massive effusion, all
the hemi-thorax can be filled and mediastinum can be shifted contra laterally. If CXR is acquired at bedside in the anterior-posterior view,
it is extremely easy to underestimate the real amount of the free
effusion ( 15). Moreover, from 10% to 25% of the milder forms of
effusion can be completely misdiagnosed by bedside CXR ( 4).
Some radiologic signs allows diagnosis of pleural effusion at
CXR, even if the classical visualization of the basal opacity is
lacking. They are the thickening of fissures and of pleural line
at the apex, the blurring of the diaphragmatic profile and the
haze of costophrenic angle, the complete but slight haze of the
hemi-thorax with still visible vascular tree. In a supine patient,
one of the more declivous part of the thorax are the apical
posterior zones, so in this place can accumulates large amount
of pleural effusion for gravity. These signs are useful only when
a comparison between the two hemi-thorax can be performed,
while in case of massive effusion equally distributed on both
sides, they are extremely difficult to be recognized. When bedside CXR is correctly interpreted, the reader can
detect large pleural effusions 92% of the time and can exclude
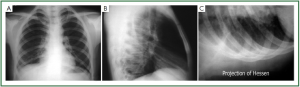
large effusions with high confidence ( 42). In selected cases a lateral view with 20° of Trendelemburg
inclination (the Hessen view) can obviate to lack of accuracy
( 37, 43) ( Figure 11A,B,C). This manoeuvre may visualize even
small amount of effusion, normally located in intrapulmonary regions, because fluid move to the pleural space near the costal
plane of the superior chest, were concavity is more accentuated.
The presence of a short pulmonary ligament allows the
accumulation of huge amount of pleural effusion (>500 mL)
below the lung, thus mimicking a lifting of the hemi-diaphragm
( Figure 12). This approach is now replaced by lung ultrasound. Of course, thoracic ultrasound has higher accuracy in the
detection of pleural effusion, and can be extremely helpful ( 35).
Another limitation of the CXR technique is the inability
to quantify the fluid collection and to diagnose the type of
effusion ( 44). Conversely, thoracic ultrasound may be helpful to
these purposes. |
|
Conclusion
In conclusion, CXR has a great potential in the first diagnosis
of many lung disorders causing acute dyspnoea and chest
pain, pending the knowledge and correct interpretation
of several signs. However, the physicians should be aware
that the sensitivity of CXR is rather low in the diagnosis
of pneumothorax, pleural effusion and pulmonary edema,
particularly in bedside-acquired images.
It has been shown a high inter-observer variability of
reading that limits the diagnostic usefulness of bedside CXR
and complicates the differential diagnosis. For these reasons it
is very important that it should be interpreted by a radiologist
experienced in thoracic radiology. Nevertheless thoracic imaging
by CXR plays a crucial role in the diagnostic process in ED,
because it allows a panoramic view, being at the same time costsafe
and relatively time-saving.
|
|
Acknowledgements
Disclosure: The authors declare no conflict of interest.
|
|
References
- Ray P, Birolleau S, Lefort Y, et al. Acute respiratory failure in the elderly:
etiology, emergency diagnosis and prognosis. Crit Care 2006;10:R82.
- Fox JC, Irwin Z. Emergency and critical care imaging. Emerg Med Clin
North Am 2008;26:787-812.
- Russell SD, Saval MA, Robbins JL, et al. New York Heart Association
functional class predicts exercise parameters in the current era. Am Heart J
2009;158:S24-30.
- Barozzi L, Valentino M. La diagnostica per immagini in pronto soccorso
Cap 4: 110-111. CG Ed Medico Scientifiche, Torino; 2008.
- Tsai TW, Gallagher E, Lombardi G, et al. Guidelines for the selective
ordering of admission chest radiography in adult obstructive airway disease.
Ann Emerg Med 1993;22:1854-8.
- Emerman CL, Cydulka R. Evaluation of high-yeld criteria for chest
radiography in acute exacerbation of chronic obstructive pulmonary
disease. Ann Emerg Med 1993;22:680-4.
- Sherman S, Skoney J, Ravikrishnan K. Routine chest radiographs in
exacerbations of chronic obstructive pulmonary disease. Arch Intern Med
1989;149:2493-6.
- Campbell SG, Murray DD, Hawass A, et al. Agreement between emergency
phisician diagnosis and radiologist reports in patients discharged from
an emergency department with community acquired pneumonia. Emerg
Radiol 2005;11:242-6.
- Eng J, Mysko WK , Weller GE, et al. Interpretation of emergency
department radiographs: a comparison of emergency medicine physicians
with radiologists, residents with faculty, and film with digital display. AJR
Am J Roentgenol 2000;175:1233-8.
- Milne ENC, Pistolesi M, Miniati M. The radiologic distinction of cardiogenic
and noncardiogenic edema. AJR Am J Roentgenol 1985;144:879-94.
- Meszaros WT. Lung changes in left heart failure. Circulation 1973;47:859-71.
- Ravin CE. Radiographic analysis of vascular distribution: a review. Bull N Y
Acad Med 1983;59:728-43.
- Mant J, Doust J, Roalfe A. Systematic review and individual patient data
meta-analysis of diagnosis of heart failure, with modelling of implications
of different diagnostic strategies in primary care. Health Technol Assess
2009;13:1-207,iii.
- Chiesa A, Olivetti L. Diagnostica per immagini in medicina clinica. CG Ed
Medico Scientifiche, Torino; 2003.
- MacMahon H. Portable chest radiology. Respir Care 1999;44:1018-32.
- Fraser RG, Parè JAP. Diagnosis of diseases of the chest. Vol 1. Ed W B
Saunders Co, Philadelphia; 1979.
- Collins SP, Lindsell CJ, Storrow AB, et al. Prevalence of negative
chest radiography results in the emergency department patient with
decompensated heart failure. Ann Emerg Med 2006;47:13-8.
- Volpicelli G, Caramello V, Cardinale L, et al. Bedside ultrasound of the lung
for the monitoring of acute decompensated heart failure. Am J Emerg Med 2008;26:585-91.
- Ruskin JA, Gurney JW, Thorsen MK, et al. Detection of pleural effusions
on supine chest radiographs. AJR Am J Roentgenol 1987;148:681-3.
- Chen JY, Chao TH, Guo YL, et al. A simplified clinical model to predict
pulmonary embolism in patients with acute dyspnea. Int Heart J
2006;47:259-71.
- Harrison A, Amudson S. Evaluation and management of the acutely
dyspnoic patient: the role of biomarkers. Am J Emerg Med 2005;23:371-8.
- Pedicelli G, Boni S, Concorsi P, et al. La tromboembolia polmonare. Radiol
Med (Torino) 1992;84:242-6.
- Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical
model to categorize patients probability of pulmonary embolism:
increasing the models utility with the simplired d-dimer. Thromb Haemost
2000;83:416-20.
- Shiber JR, Santana J. Dyspnea. Med Clin North Am 2006;90:453-79.
- Fox JC, Irwin Z. Emergency and critical care imaging. Emerg Med Clin
North Am 2008;26:787-812.
- Westcott J. Criteria of appropriateness for dyspnea. Radiology
2000;215:641-643.
- Fleischner FG. Observation of the radiologic changes in pulmonary
embolism - In: Sasahara AA (Ed) “Pulmonary Embolic Disease”, New York;
1965.
- Heitzman ER. The lung: radiologic pathologic correlation. CV Mosby
Company. St Louis; 1984.
- Tocino IM, Miller MH, Fairfax WR. Distribution of pneumothorax in
the supine and semirecumbent critically ill adult. AJR Am J Roentgenol
1985;144:901-5.
- Weissberg D, Refaely Y. Pneumothorax: experience with 1,199 patients.
Chest 2000;117:1279-85.
- Kelley JM, Elliott PL. The radiologic evaluation of patient with suspected
pulmonary thromboembolic disease. Med Clin North Am 1975;59:3-36.
- Moses DC, Silver TM, Bookstein JJ. The complementary roles of chest
radiography, lung scanning and selective pulmonary angiography in the
diagnosis of pulmonary embolism. Circulation 1974;49:179-88.
- Worsley DF, Alavi A, Aronchic JM, et al. Chest radiographic findings in
patients with acute pulmonary embolism: observations from the PIOPED
study. Radiology 1993;189:133-6.
- Westermark N. On the Roentgen diagnosis of lung embolism. Acta Radiol
1938;19:357-72.
- Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based
recommendations for point-of-care lung ultrasound. Intensive Care Med
2012;38:577-91.
- Volpicelli G. Sonographic diagnosis of pneumothorax. Intensive Care Med
2011;37:224-32.
- Trupka A, Waydhas C, Hallfeldt KK, et al. Value of thoracic computed
tomography in the first assessment of severely injured patients with blunt
chest trauma: results of a prospective study. J Trauma 1997;43:405-11.
- Khan AN, Al-Jahdali H, Al-Ghanem S, et al. Reading chest radiographs
in the critically ill (part I): normal chest radiographic appearance,
instrumentations and complication from instrumentation. Ann Thorac
Med 2009;4:75-87.
- Light RW, George RB. Incidence and significance of pleural effusion after
abdominal surgery. Chest 1976;69:621-5.
- Muller R, Lofstedt S. The reacting of the pleura in primary tuberculosis of
the lungs. Acta Med Scand 1945;122:105-33.
- Hessen I. Roentgen examination of pleural fluid: a study of the localization of
free effusion, the potenzialities of diagnosing minimal quantities of fluid and
its existence under physiological conditions. Acta Radiol 1951;86:1-80.
- Kitazono MT, Lau CT, Parada AN, et al. Differentiation of pleural effusions
from parenchymal opacities: accuracy of bedside chest radiography. AJR
Am J Roentgenol 2010;194:407-12.
- Garofalo G, Busso M, Perotto F, et al. Ultrasound diagnosis of
pneumothorax. Radiol Med (Torino) 2006;111:516-25.
- Eibenberger KL, Dock WI, Ammann ME, et al. Quantification of pleural
effusions: sonography versus radiography. Radiology 1994;191:681-4.
Cite this article as: Cardinale L, Volpicelli G, Lamorte
A, Martino J, Veltri A. Revisiting signs, strengths and
weaknesses of Standard Chest Radiography in patients
of Acute Dyspnea in the Emergency Department.
J Thorac Dis 2012;4(4):398-407. doi: 10.3978/
j.issn.2072-1439.2012.05.05
|