Diameter and growth rate of the thoracic aorta—analysis based on serial computed tomography scans
Introduction
Establishing reference values of aortic size is not only a crucial component in clinical decisions in aortic diseases but can also be used as baseline data for assessing the risk of other cardiovascular diseases (1-3). Therefore, many studies have investigated the average aortic size in the general population and its relationship with demographic and anthropometric parameters. Many of them were based on autopsy data or transthoracic echocardiographic examination (4-7). Studies using computed tomography (CT) scans or magnetic resonance imaging investigated relatively small cohorts (8,9).
Previous studies reported that the size of the thoracic aorta is correlated with age, sex, and body size (6,10,11). However, the detailed features of thoracic aortic growth accompanying the aging process have not been well elucidated because previous studies were based on single-time cross-sectional observations with a small cohort size. Unlike the previous studies, each subject in this study underwent consecutive CT scans. The size of the thoracic aorta was measured, and the long-term growth rate was calculated based on these serial CTs, which were taken at sufficient intervals to enable these calculations. The first objective of this study was to investigate whether the correlation of aortic size with age, sex, and body surface area (BSA) is consistently shown in the entire thoracic aorta. The second objective was to analyze the factors influencing the growth rate of the thoracic aorta. We present this article in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-1275).
Methods
Study subjects
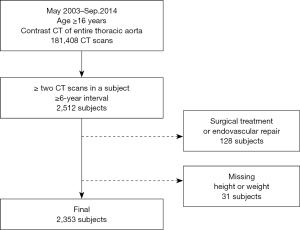
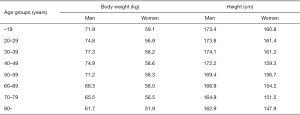
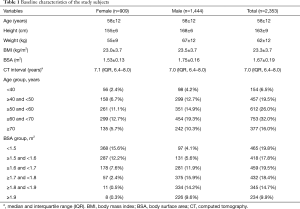
In this retrospective observational study, data were collected through a review of electronic medical records. The institutional review board of Seoul National University Bundang Hospital approved this study and waived the need for informed consent owing to its retrospective nature without disclosing patients’ private information (No. B-1909-567-104). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization. We performed a query on the clinical data warehouse based on the electronic medical records of our institution to determine all CT examinations that visualized the entire thoracic aorta with contrast enhancement in adult patients (≥18 years). The CT protocols included in this study were as follows; (I) CT angiography + 3D coronary, thoracic aorta, (II) CT angiography + 3D thoracic aorta and branches, (III) CT angiography + 3D coronary, chest and (IV) Chest CT (contrast) + 3D (esophagus), (V) Chest CT (contrast) + 3D (breast), (VI) Chest CT (contrast) + 3D (Thoracic Surgery), (VII) Chest CT (contrast, for navigation bronchoscopy), (VIII) Chest CT - Limited Contrast, and (IX) CT angiography + 3D Adamkiewicz artery (thoracoabdominal aorta) were included. Data on 181,408 CT scans from May 2003 to September 2014 met these criteria, of which 28,032 patients underwent two or more CT scans in the same period. The criteria were further narrowed down to identify 2,512 patients who had an interval of 6 years or longer between the first and last CT scans. Among these, patients who underwent surgical or endovascular repair of the thoracic aorta (n=128) and those whose height or weight were missing from the medical records (n=31) were excluded (Figure S1). Finally, 2,353 patients were enrolled in this study, and their CT images were reviewed. All the subjects were Koreans and their characteristics are shown in Table 1. BSA was calculated using the Du Bois formula (BSA =0.007184 × W0.425 × H0.725) (12). For reference, mean body weight and height of general Korean population are presented in Figure S2.
Full table
Aortic size measurement
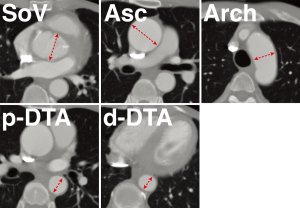
To eliminate inter-observer differences, one of the authors (HA) undertook the review of all CT images. Aortic diameter was measured on the transverse axial images of the first and last CT scans at 5 levels: sinus of Valsalva, ascending aorta, aortic arch, proximal descending thoracic aorta (DTA), and distal DTA (Figure 1). The size of the sinus of Valsalva was measured at the level of maximum dimension found on axial imaging, and the ascending aortic diameter was measured as the short diameter at the level of the pulmonary artery bifurcation. The aortic arch short diameter was measured on the axial section that showed the aortic arch to the maximal extent while the proximal DTA was measured at the same level as the main pulmonary artery. In addition, the distal DTA was measured at the cardiac apex level while the internal diameter excluding the thickness of the aortic wall, i.e., contrast-enhanced luminal diameter, was recorded for analysis. The growth rate was calculated by dividing the difference of the diameters by the interval between the first and last CT examinations. Indexed aorta diameter was defined as aortic diameter divided by BSA.
Statistical analyses
Statistical analyses were performed using statistics software R (R 3.6.1; The R Foundation for Statistical Computing, Vienna, Austria). For basic analyses, an independent samples t-test was performed. To analyze the correlation between dependent variables (aortic size at 5 levels) and independent variables (age, BSA), univariable linear regression was carried out. Multivariable linear regressions were also utilised to model aortic size from age, sex, and BSA. The growth rate was compared by age groups using the Kruskal-Wallis test. Further, univariable linear regression was used to model growth rate divided by the quintiles of the indexed aortic diameter. For all linear regressions, the estimated coefficient (β) with 95% confidence intervals and R2 were shown. A P value less than 0.05 was considered statistically significant.
Results
Initial aortic diameter
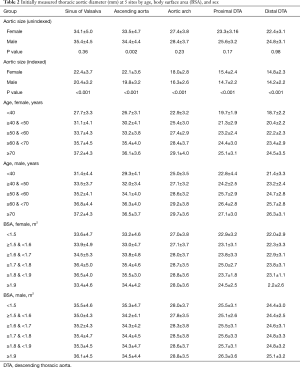
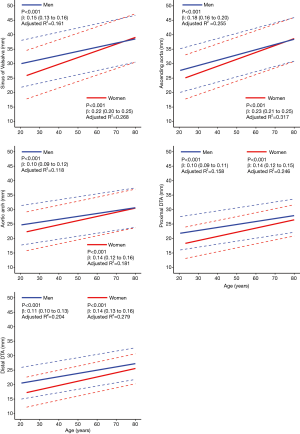
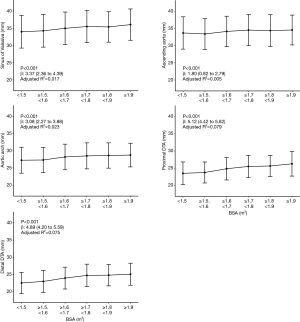
The mean diameters of the aorta were 34.9±4.7, 34.1±4.6, 28.0±3.8, 24.8±3.4, and 23.8±3.3 mm at the sinus of Valsalva, ascending aorta, arch, proximal DTA, and distal DTA, respectively. The aortic diameter was larger in men than in women with statistical significance only in the ascending aorta, whereas the indexed diameter (diameter divided by BSA) was significantly larger in women at all five levels (Table 2). Older subjects had a larger aortic diameter at all five levels (Figure 2). Regardless of sex, all segments of the thoracic aorta for subjects in their 70s were 5 to 10 mm larger than that of patients in their 30s. The R2 values of these univariable regressions, which represent how close the data are to the fitted regression line, were 0.118 to 0.317. Moreover, a larger BSA was associated with a larger aortic diameter at all five levels (Table 2, Figure 3), although R2 was relatively smaller (0.005 to 0.079). Multivariable linear regression adjusted by sex showed that age and BSA were independent predictors and were positively correlated with aortic diameter at all five levels (Table 3). Multivariable models had R2 values of 0.212 to 0.393.
Full table

Full table
Growth rate of the thoracic aorta
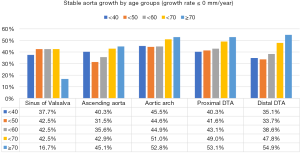
A substantial number of patients showed no growth or a negative value (stable aortic growth) during the interval as follows: sinus of Valsalva (n=995, 42%), ascending aorta (n=917, 39%), aortic arch (n=1,133, 48%), proximal DTA (n=1,087, 46%), and distal DTA (n=1,011, 43%) (Figure S3). The median growth rates of the sinus of Valsalva were 0.15 [interquartile range (IQR) mm/year: −0.12 to 0.42]; ascending aorta, 0.15 (IQR: 0.00 to 0.32) mm/year; aortic arch, 0.12 (IQR: −0.13 to 0.30) mm/year; proximal DTA, 0.12 (IQR: 0.00–0.28) mm/year; and distal DTA, 0.13 (IQR: 0.00–0.30) mm/year.
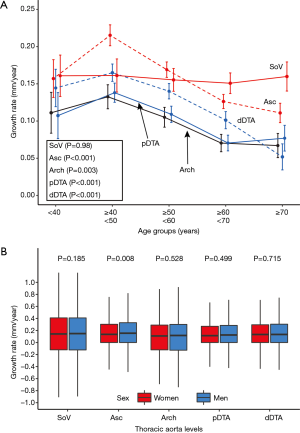
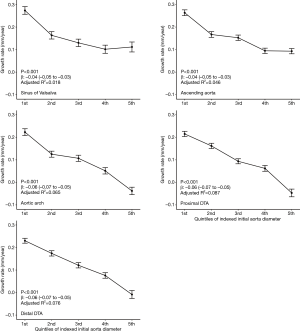
At all five levels, the mean growth rate was the highest in subjects in their 40s (Figure 4A). The Kruskal-Wallis test showed a significant difference in the growth rate according to age groups in the ascending aorta, aortic arch, proximal DTA, and distal DTA. The growth rate of the sinus of Valsalva did not differ significantly among age groups (P=0.98). As for the sex difference in the aortic growth rate, it was significantly higher in male subjects only at the ascending aorta, and no difference was found at the other levels (Figure 4B). The indexed initial aortic diameter was divided into 5 groups (quintiles) to analyze the relationship between the initial aortic size and later growth rate. The growth rate gradually decreased from the first to the fifth quintile, and the trend was statistically significant in univariable linear regression analysis at all five levels (Figure 5).
Discussion
Consistent with previous studies, we found that the diameter of the thoracic aorta correlated positively with age, sex, and body size (4,6,10,11). In particular, the correlation with age was relatively stronger with R2 of 0.118 to 0.317. The multivariable linear regression model including age, sex, and BSA showed a slightly higher R2 of 0.212 to 0.393, which is similar to the results of a previous study (4). However, an R2 less than 0.5 still indicates that the biological variability of aortic size is not sufficiently explained by these simple variables. A large population-based study similar to our investigation was previously conducted in Germany (13). Measurement by non-contrast CT in 4,129 residents (aged 45 to 75 years) of the Ruhr area revealed that the average diameter of the thoracic aorta increases by 1.5 to 1.7 mm every 10 years. The average diameters of the ascending and DTA were 3.71 and 2.82 cm in men and 3.45 and 2.54 cm in women, respectively. Those values are approximately 3 to 4 mm larger than the equivalents in our patients, who had a smaller mean BSA than the German population by almost 0.3 m2. In contrast, the BSA-adjusted diameters are larger in the Korean population, and the difference is more remarkable in the ascending aorta, especially in female subjects (22.1 mm/m2 in Koreans vs. 19.3 mm/m2 in Germans). A few studies regarding the difference in aortic diameter according to ethnicity suggested that Asians have a larger indexed aortic diameter, whereas African Americans have smaller values than Caucasians (9,14,15). As for sex differences, our result is in line with previous studies in which women had a larger aortic diameter adjusted by body size (13,16). Consequently, the common findings of this present and previous studies indicated that women and Asians tend to have a larger thoracic aorta relative to their body size. Further, age and body size are the strongest factors affecting absolute aortic diameter.
In the literature, the rate of aortic expansion is about 0.7 to 1.7 mm for each decade (13,16). Our results showed a similar aortic growth rate, which was faster in the ascending aorta than in the distal segments. The rate reached the highest value in patients in their 40s and then decreased with further aging. This finding is in line with the report of Biaggi et al. who speculated that the highest growth rate appears early in adulthood when the elastic lamellae are mostly intact and collagen fibers are relatively sparse (17). The relative growth spurt in the 40s might also be explained by the abrupt increase in aortic stiffness in the later period (16). Another important finding is that a larger initial indexed diameter was associated with a lower growth rate. This finding may be discordant with a previous belief that growth is faster in the larger aorta (18-20). Such a belief might have been based on biased observations of patients with aortic diseases. Our observation makes us infer that aging-related aortic dilatation slows down beyond middle age in the general hospital population. It may also imply that control of risk factors for aortic aneurysm, such as hypertension, is better to be started in early adulthood.
The present study has two important strengths. First, it was conducted on a relatively large group of subjects who underwent CT examinations for various reasons including comprehensive medical check-ups for those in an apparently healthy condition. Those who had aortic diseases indicating surgical or endovascular intervention were excluded. Consequently, compared with the characteristics of subjects in previous studies that had a small sample size and a narrow age range, the characteristics of our study subjects are considered to be more similar to those of the general population in the middle and older age range (11,21,22). Second, contrast-enhanced CT scans, which are regarded to be more accurate in measuring the aortic diameter than non-contrast CT or transthoracic echocardiography, were reviewed. Many previous studies used echocardiography as the examination tool, which has limitations such as inter-observer variation, inferior spatial resolution, and inaccuracy in examining the aortic arch and other segments beyond.
To the best of our knowledge, this study is the first to investigate the change of aortic diameter over a long period of time by utilizing serial CT scans from the same individuals. An important finding of this unique study is that the aortic diameter remained stable for many years in more than 40% of the study subjects, suggesting that aging-related aortic dilatation is neither uniform in all people nor is a linear persistent process. Such a result has not been found by previous studies, the majority of which were cross-sectional observational studies that deduced the aortic growth rate from the difference between age groups.
This study was enabled by a large database called a clinical data warehouse, which contains the results of all laboratory and imaging examinations performed in our hospital. However, the database is different from pre-planned registries and lacks comprehensive information of illness and medication history of patients. Consequently, to our regret, we did not proceed to the labor-intensive, if not impossible, investigation of the factors that may affect aortic growth, such as familial history, hypertension, metabolic disorders, and chronic renal diseases. Moreover, potentially confounding medications such as beta blockers, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers were not checked. In addition, a few more limitations can be pointed out. First, although we assumed that our study subjects were similar to the general healthy population, a substantial proportion had non-aortic but major illnesses, some of which may affect aortic dilatation. Second, ‘regression to the mean’ should be considered when interpreting our data. There is a possibility that a lesser growth rate in the larger aorta and a greater growth rate in the smaller aorta could be related to the regression to the mean. Third, the CT equipment and imaging protocols in many patients were different between the two images analyzed because of the difference in the reasons for CT scanning and the acquisition of new scanners during the study period. The aorta size was measured on axial scans rather than on reconstructed images. Additionally, most of the CT scans were not electrocardiographically gated. This might also lead to inconsistency in measuring the aortic diameter. Finally, as few patients in our study population were under 40 years old, we could not have insight into the features of aortic growth in early adulthood. Considering that CT examination is rarely performed in young people, the collection of such data would be almost impossible in retrospective studies of this kind. In a long-term prospective observation of a large cohort aged 23–35 years, the average aortic root diameter increases by 3 mm during a 20-year interval (15). However, that study was based on echocardiography that enabled the observation of only the aortic root.
In conclusion, the thoracic aorta is generally dilated with aging and was larger in subjects with a larger body size. Sex difference in the gross aortic diameter appeared to be associated with a difference in body size. In those who did not have significant aortic disease, aortic growth was faster in younger subjects with a smaller indexed diameter.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1275
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-20-1275
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1275
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1275). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization. The institutional review board of Seoul National University Bundang Hospital approved this study and waived the need for informed consent owing to its retrospective nature without disclosing patients’ private information (No. B-1909-567-104).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kim SY, Martin N, Hsia EC, et al. Management of aortic disease in Marfan Syndrome: a decision analysis. Arch Intern Med 2005;165:749-55. [Crossref] [PubMed]
- Gardin JM, Arnold AM, Polak J, et al. Usefulness of aortic root dimension in persons > or = 65 years of age in predicting heart failure, stroke, cardiovascular mortality, all-cause mortality and acute myocardial infarction (from the Cardiovascular Health Study). Am J Cardiol 2006;97:270-5. [Crossref] [PubMed]
- Evangelista A, Flachskampf FA, Erbel R, et al. Echocardiography in aortic diseases: EAE recommendations for clinical practice. Eur J Echocardiogr 2010;11:645-58. [Crossref] [PubMed]
- Saura D, Dulgheru R, Caballero L, et al. Two-dimensional transthoracic echocardiographic normal reference ranges for proximal aorta dimensions: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging 2017;18:167-79. [Crossref] [PubMed]
- Mirea O, Maffessanti F, Gripari P, et al. Effects of aging and body size on proximal and ascending aorta and aortic arch: inner edge-to-inner edge reference values in a large adult population by two-dimensional transthoracic echocardiography. J Am Soc Echocardiogr 2013;26:419-27. [Crossref] [PubMed]
- Devereux RB, de Simone G, Arnett DK, et al. Normal limits in relation to age, body size and gender of two-dimensional echocardiographic aortic root dimensions in persons ≥15 years of age. Am J Cardiol 2012;110:1189-94. [Crossref] [PubMed]
- Ninomiya OH, Tavares Monteiro JA, Higuchi Mde L, et al. Biomechanical Properties and Microstructural Analysis of the Human Nonaneurysmal Aorta as a Function of Age, Gender and Location: An Autopsy Study. J Vasc Res 2015;52:257-64. [Crossref] [PubMed]
- Wolak A, Gransar H, Thomson LE, et al. Aortic size assessment by noncontrast cardiac computed tomography: normal limits by age, gender, and body surface area. JACC Cardiovasc Imaging 2008;1:200-9. [Crossref] [PubMed]
- Turkbey EB, Jain A, Johnson C, et al. Determinants and normal values of ascending aortic diameter by age, gender, and race/ethnicity in the Multi-Ethnic Study of Atherosclerosis (MESA). J Magn Reson Imaging 2014;39:360-8. [Crossref] [PubMed]
- Agmon Y, Khandheria BK, Meissner I, et al. Is aortic dilatation an atherosclerosis-related process? Clinical, laboratory, and transesophageal echocardiographic correlates of thoracic aortic dimensions in the population with implications for thoracic aortic aneurysm formation. J Am Coll Cardiol 2003;42:1076-83. [Crossref] [PubMed]
- Muraru D, Maffessanti F, Kocabay G, et al. Ascending aorta diameters measured by echocardiography using both leading edge-to-leading edge and inner edge-to-inner edge conventions in healthy volunteers. Eur Heart J Cardiovasc Imaging 2014;15:415-22. [Crossref] [PubMed]
- Du BOIS D. Du BOIS EF. Clinical Calorimetry: Tenth paper - A formula to estimate the approximate surface area if height and weight be known. JAMA Internal Medicine 1916;XVII:863-71.
- Kälsch H, Lehmann N, Mohlenkamp S, et al. Body-surface adjusted aortic reference diameters for improved identification of patients with thoracic aortic aneurysms: results from the population-based Heinz Nixdorf Recall study. Int J Cardiol 2013;163:72-8. [Crossref] [PubMed]
- Laughlin GA, Allison MA, Jensky NE, et al. Abdominal aortic diameter and vascular atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Eur J Vasc Endovasc Surg 2011;41:481-7. [Crossref] [PubMed]
- Teixido-Tura G, Almeida AL, Choi EY, et al. Determinants of Aortic Root Dilatation and Reference Values Among Young Adults Over a 20-Year Period: Coronary Artery Risk Development in Young Adults Study. Hypertension 2015;66:23-9. [Crossref] [PubMed]
- Vriz O, Driussi C, Bettio M, et al. Aortic root dimensions and stiffness in healthy subjects. Am J Cardiol 2013;112:1224-9. [Crossref] [PubMed]
- Biaggi P, Matthews F, Braun J, et al. Gender, age, and body surface area are the major determinants of ascending aorta dimensions in subjects with apparently normal echocardiograms. J Am Soc Echocardiogr 2009;22:720-5. [Crossref] [PubMed]
- Yiu RS, Cheng SW. Natural history and risk factors for rupture of thoracic aortic arch aneurysms. J Vasc Surg 2016;63:1189-94. [Crossref] [PubMed]
- Dapunt OE, Galla JD, Sadeghi AM, et al. The natural history of thoracic aortic aneurysms. J Thorac Cardiovasc Surg 1994;107:1323-32; discussion 32-3. [Crossref] [PubMed]
- Coady MA, Davies RR, Roberts M, et al. Familial patterns of thoracic aortic aneurysms. Arch Surg 1999;134:361-7. [Crossref] [PubMed]
- Son MK, Chang SA, Kwak JH, et al. Comparative measurement of aortic root by transthoracic echocardiography in normal Korean population based on two different guidelines. Cardiovasc Ultrasound 2013;11:28. [Crossref] [PubMed]
- Fitzgerald BT, Kwon A, Scalia GM. The New Dimension in Aortic Measurements - Use of the Inner Edge Measurement for the Thoracic Aorta in Australian Patients. Heart Lung Circ 2015;24:1104-10. [Crossref] [PubMed]