
Combining immunotherapy and radiotherapy in lung cancer: a promising future?
Lung cancer remains the leading cause of cancer incidence and mortality, with an estimated over 2 million new cases and 1.8 million deaths worldwide in 2018 (1). The number of deaths due to lung cancer in 2018 was close to one-fifth of all cancer deaths (1). The main pathological types of lung cancer include non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC), and NSCLC accounts for approximately 80–85% of all cases (2). The treatment of NSCLC is stage-specific. For patients with stage I or II disease, complete surgical resection should be performed if there are no contraindications. Patients with unresectable disease should be considered for chemotherapy with/without radiotherapy. For a long time, platinum-based chemotherapy has been the main option for the first-line treatment of metastatic NSCLC patients (3). Since the 1990s, with the understanding of lung cancer driver genes, targeted therapies [including but not limited to drugs targeting epidermal growth factor receptor (EGFR) mutations, KRAS mutations, ALK gene rearrangements and ROS1 rearrangements] have changed the treatment of NSCLC with driver gene mutations, which has significantly prolonged survival (4,5). However, despite considerable progress and the development of new drugs, the estimated 5-year overall survival (OS) rate remains at 18% (6). Thus, there is an urgent need to develop valid NSCLC therapies with low toxicity.
In 2011, the immune checkpoint inhibitor (ICI) ipilimumab, an anti-cytotoxic T lymphocyte-associated protein 4 (CTLA-4) antibody, was approved for the treatment of metastatic melanoma, resulting in a revolution in cancer treatment (7). To date, ICIs, mainly targeting programmed death 1 (PD-1), programmed death-ligand 1 (PD-L1), or CTLA-4, are transforming treatment strategies for multiple cancer types, especially advanced NSCLC (8,9). Radiotherapy has also developed rapidly in the past two decades, especially stereotactic body radiation therapy (SBRT), which is now widely accepted as an alternative primary treatment for NSCLC patients who are not suitable for surgery (10). Currently, there are a number of clinical trials on immunotherapies alone or in combination with other treatments for lung cancers, and radiotherapy combined with immunotherapy has attracted increasing attention (10). Table 1 shows a summary of ongoing randomized studies combining immunotherapies and radiotherapy in lung cancer. According to current clinical trial data, radiotherapy combined with immunotherapy can significantly improve the survival of lung cancer patients without significantly increasing adverse reactions (9,11,12).
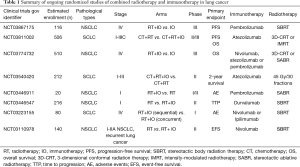
Full table
Shaverdian et al. (11) confirmed that the combination of radiotherapy and pembrolizumab for the treatment of advanced NSCLC can prolong progression-free survival (PFS) and OS with an acceptable safety profile in KEYNOTE-001, a phase I trial. Compared with the patients who received pembrolizumab only, the patients who received radiotherapy as a precondition prior to pembrolizumab showed a significantly longer PFS (4.4 vs. 2.1 months) and OS (10.7 vs. 5.3 months). There was no significant difference in the frequency of any pulmonary toxicity or the frequency of grade 3 or worse pulmonary toxicity between patients who had received previous thoracic radiotherapy and those who did not (11). Theelen et al. (12) found similar results in a randomized phase II trial, PEMBRO-RT. Seventy-six patients with metastatic NSCLC enrolled in the study were randomly assigned in a 1:1 ratio to receive pembrolizumab without (control arm) or with (experimental arm) SBRT to a single metastatic site within 7 days before immunotherapy. The experimental arm showed an increase in the overall response rate (18% vs. 36%; P=0.07), disease control rate (40% vs. 64%; P=0.04) at 12 weeks, median PFS (1.9 vs. 6.6 months) and median OS (7.6 vs. 15.9 months), as compared with the control arm. The increased PFS and OS in the experimental arm was not statistically significant. The most common adverse events were fatigue, flu-like symptoms, and cough, among which fatigue (27% vs. 51%; P=0.05) and pneumonia (8% vs. 26%; P=0.06) occurred more often in the experimental arm than in the control arm. Grade 3 to 5 pembrolizumab-related toxic effects were reported in 12 patients (17%), with no significant difference between the experimental arm and the control arm. The combination of radiotherapy and immunotherapy appeared to be very well tolerated.
The well-known PACIFIC trial conducted by SJ Antonia is a recent phase III randomized trial designed to compare durvalumab (an anti-PD-L1 antibody) with placebo in stage III unresectable NSCLC patients who did not progress after 2 or more cycles of definitive concurrent platinum-based chemoradiotherapy (9). A total of 713 patients were enrolled in the study, and were randomized in a 2:1 ratio to receive durvalumab (within 1–42 days after receiving concurrent chemoradiation) or placebo, with stratification factors including age (<65 vs. ≥65 years), sex and smoking history (current or former smoker vs. never smoker). Most patients were current or former smokers and did not have EGFR mutations, and their PD-L1 status was typically less than 25% or unknown. The median PFS from randomization was 16.8 months for the durvalumab group vs. 5.6 months for the placebo group (hazard ratio, 0.52; 95% CI, 0.42–0.65; P<0.001), with a three-fold increase. The response rate was significantly higher in the durvalumab group than in the placebo group (28.4% vs. 16.0%; P<0.001) (9). The 24-month OS rate was 66.3% (95% CI, 61.7–70.4) in the durvalumab group vs. 55.6% (95% CI, 48.9–61.8) in the placebo group (P=0.005) (13); the median time to death or distant metastasis was 28.3 vs. 16.2 months. Durvalumab significantly prolonged OS, compared with placebo (hazard ratio, 0.68; 99.73% CI, 0.47–0.997; P=0.0025). In terms of safety, 96.8% of the patients in the durvalumab group and 94.9% of those in the placebo group had adverse events of any cause and grade, and grade 3 or 4 adverse events occurred in 29.9% and 26.1%, respectively (9). However, 15.4% and 9.8% of the patients in the durvalumab group and placebo group, respectively, discontinued the trial regimen because of adverse events. There were no significant differences in the incidences of pneumonitis (4.8% vs. 2.6%) and radiation pneumonitis (1.3% vs. 1.3%), which were the most frequent adverse events leading to the discontinuation of the trial regimen. Therefore, the addition of durvalumab did not significantly increase the toxicity. The results indicated that combining radiotherapy and immunotherapy is effective and well tolerated for advanced NSCLC patients. Based on the breakthrough result of the PACIFIC trial, the US Food and Drug Administration (FDA) approved durvalumab for the treatment of advanced NSCLC without progression after platinum-based chemoradiation therapy in July 2017.
The efficacy and safety of radiotherapy combined with immunotherapy for lung cancer treatment has been confirmed according to these studies, but the best treatment regimen for combining radiotherapy and ICIs is currently unclear, and this may significantly affect the generation of a durable therapeutic antitumor immune response. A preclinical study by Dovedi et al. showed that the simultaneous delivery of radiotherapy and immunotherapy is superior to their sequential delivery (14). They evaluated three distinct combination schedules in which mice with colon carcinoma CT26 tumors established by subcutaneous inoculation received local radiotherapy with immunotherapy. Administration of the αPD-L1 mAb was initiated on the first day of fractionated radiation therapy (schedule A), on the fifth day of radiation therapy (schedule B), or 7 days after the completion of radiation therapy (schedule C). The results showed that the OS rates were significantly higher for both schedules A and B than for schedule C, and there was no significant difference in OS between schedules A and B, with long-term survival (LTS) rates of 60% and 57%, respectively (P>0.05) (14).
In 1953, Mole noticed tumor regression located far outside the radiation field in a patient receiving radiotherapy to a single site of metastatic disease, and he first described this phenomenon as an “abscopal effect” (15). This phenomenon was not commonly observed in the following decades, until ipilimumab was approved for the treatment of metastatic melanoma in 2011, and immunotherapy began to be used clinically. These “abscopal effects” were observed in an increasing number of patients receiving radiotherapy with or without immunotherapy (16). Since then, a large number of clinical trials have been conducted to evaluate the safety and effectiveness of radiotherapy combined with immunotherapy, almost all of which use this single-site irradiation. However, Brooks et al. (17) demonstrated that radiation therapy should be delivered to all targetable disease sites instead of a single site when combined with immunotherapy, which was supported by both clinical data and biological rationale. Preclinical studies have revealed that radiation improves the ability of the immune system to recognize solid tumors by uncovering or releasing previously hidden tumor-associated antigens (TAAs) and immunostimulatory molecules from within the tumor that can activate and prime an antitumor immune response (Figure 1) (17-19). Radiotherapy for all targetable disease sites increases the unveiling of TAAs and hence increases the possibility of eliciting an immune response successfully by TAAs. In addition, multifocal radiotherapy may overcome the obstacle of tumor heterogeneity because it may induce immune cells to recognize a wider range of TAAs and may also destroy or enhance the destruction of resistant subclones, which may reduce the complete response rate of ICI therapy. In addition, radiotherapy for all targetable disease sites stimulates the tumor vasculature and has negative feedback immunosuppressive properties for large tumors, providing optimal conditions for immune cells to enter the entire tumor, thereby enhancing the osmotic effect on tumors and overcoming the inhibition of the antitumor immunosuppressive effect of the immune response (17).
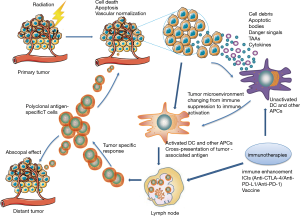
Studies in patients with advanced solid tumors have shown that a T cell-inflamed tumor microenvironment has a more favorable response to immunotherapy (20,21). Tumors with a high proportion of tumor-infiltrating lymphocytes (TILs) are often referred to as ‘hot’ tumors and are associated with a better prognosis (20). ‘Cold’ tumors describe a phenotype of solid tumors with scanty T lymphocytes, which may not benefit from ICIs (21). Radiotherapy can induce DNA damage and then result in cell death. During tumor cell death induced by ionizing radiation, tumor antigens are released and presented to dendritic cells and other antigen-presenting cells, which activate the adaptive immune system, stimulate the proliferation of T cells and finally initiate a tumor-specific response (17,22,23). In this way, radiation can prime the innate immune system, recruiting T lymphocytes and turning ‘cold’ tumors into ‘hot’ tumors, which are more likely to respond to systemic immunotherapy (23). Similarly, Bernstein et al. (18) suggested that SABR results in immune activation by inducing tumor cell death, modulating the tumor cell phenotype and normalizing aberrant tumor vasculature. After cell death, tumor debris carrying associated danger signals, TAAs, and inflammatory cytokines are recognized by dendritic cells and other antigen-presenting cells, promoting antigen presentation to the cells of the immune system. Then, polyclonal antigen-specific T cells are generated, some of which can attack tumors located in the radiation field as well as distant tumors (Figure 1) (24).
Preclinical studies also described the synergistic activity between radiation and PD-1 inhibition. Herter-Sprie et al. (25) observed significant tumor regression of the target lesion 4 weeks after combined radiotherapy and immunotherapy, and this remained stable with very low tumor burden 12 weeks after the end of treatment in Kras mice. To further explore how radiotherapy affects the quantity and subsequent recruitment of tumor-associated immune cell populations, they used flow cytometry to analyze the total numbers of tumor-infiltrating lymphoid and myeloid cell populations before and after radiotherapy. The results showed a significant decline in all lymphoid populations 24 hours after radiotherapy, indicating their radiosensitivity. Studies of cell count at 96 hours after radiotherapy showed that B, NK and T cell subsets began to increase, although only NK cells were no longer significantly reduced compared to that in naive tumors (25). They also observed a 65% reduction in the T regulatory (Treg) cell population in Kras tumors after radiotherapy, which caused an increase in the CD8+/Treg ratio 96 hours after radiotherapy (25). Lazzari et al. (3) suggested that radiotherapy might favor the release of TAAs, which stimulate a systemic immune response. Furthermore, radiotherapy might reduce the number of Tregs, upregulate MHC class I molecules on cancer cells, and further induce immunogenic cell death. These effects stimulate the recruitment and maturation of dendritic cells as well as other antigen-presenting cells, and promote antigen presentation by inducing the release of antigens and damage-associated molecular patterns.
Mounting preclinical evidence indicates that the synergistic effect of radiotherapy combined with immunotherapy can enhance the tumor killing effect. The safety and efficacy of the strategy of combining radiotherapy and immunotherapy in the treatment of NSCLC was verified by several clinical trials as described above. In the near future, ICIs alone or in combination with radiotherapy, chemotherapy or other immunotherapy may become the standard care in the first-line treatment of advanced NSCLC patients without driver genes. Although radiotherapy combined with immunotherapy has made a breakthrough in the treatment of NSCLC, it is urgent to identify the population who may best benefit from combination therapy in clinical applications because there is currently no single biomarker that can serve as the sole marker of a radiotherapy-induced immune response to accurately evaluate the immune response to radiotherapy. The effects of radiotherapy on the tumor microenvironment are becoming clearer, but the interaction between radiotherapy and immunization may be much more complex, and more pathways involving interactions may not have been discovered yet. Preclinical and clinical trials are urgently needed to further clarify these unanswered questions, including the use of biomarkers, the synergy between radiotherapy and immunotherapy, the optimal schedule for combining radiotherapy, and the sequence of the combination of radiotherapy and immunotherapy. We are convinced that we are entering an exciting era for transforming cancer into a chronic disease in the near future.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Peng Luo, Clare Y. Slaney and Jian Zhang) for the series “Immunotherapy and Tumor Microenvironment” published in Journal of Thoracic Disease. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/JTD-2019-ITM-001). The series “Immunotherapy and Tumor Microenvironment” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Villaruz LC, Kalyan A, Zarour H, et al. Immunotherapy in lung cancer. Transl Lung Cancer Res 2014;3:2-14. [PubMed]
- Lazzari C, Karachaliou N, Bulotta A, et al. Combination of immunotherapy with chemotherapy and radiotherapy in lung cancer: is this the beginning of the end for cancer? Ther Adv Med Oncol 2018;10:1758835918762094. [Crossref] [PubMed]
- Duma N, Santana-Davila R, Molina JR. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin Proc 2019;94:1623-40. [Crossref] [PubMed]
- Cafarotti S, Lococo F, Froesh P, et al. Target Therapy in Lung Cancer. Adv Exp Med Biol 2016;893:127-36. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Sharma P, Wagner K, Wolchok JD, et al. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer 2011;11:805-12. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Walker J, Loo BW Jr. Radiotherapy and Immunotherapy-Shining Further Together. JAMA Oncol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 2017;18:895-903. [Crossref] [PubMed]
- Theelen WSME, Peulen HMU, Lalezari F, et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol 2019;5:1276-82. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res 2014;74:5458-68. [Crossref] [PubMed]
- Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol 1953;26:234-41. [Crossref] [PubMed]
- Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer 2016;40:25-37. [Crossref] [PubMed]
- Brooks ED, Chang JY. Time to abandon single-site irradiation for inducing abscopal effects. Nat Rev Clin Oncol 2019;16:123-35. [Crossref] [PubMed]
- Bernstein MB, Krishnan S, Hodge JW, et al. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat Rev Clin Oncol 2016;13:516-24. [Crossref] [PubMed]
- Grass GD, Krishna N, Kim S. The immune mechanisms of abscopal effect in radiation therapy. Curr Probl Cancer 2016;40:10-24. [Crossref] [PubMed]
- Gajewski TF. The Next Hurdle in Cancer Immunotherapy: Overcoming the Non-T-Cell-Inflamed Tumor Microenvironment. Semin Oncol 2015;42:663-71. [Crossref] [PubMed]
- Ji RR, Chasalow SD, Wang L, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother 2012;61:1019-31. [Crossref] [PubMed]
- Schuler G, Steinman RM. Dendritic cells as adjuvants for immune-mediated resistance to tumors. J Exp Med 1997;186:1183-7. [Crossref] [PubMed]
- Bhalla N, Brooker R, Brada M. Combining immunotherapy and radiotherapy in lung cancer. J Thorac Dis 2018;10:S1447-60. [Crossref] [PubMed]
- Oliver AJ, Darcy PK, Trapani JA, et al. Cross-talk between tumors at anatomically distinct sites. FEBS J 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Herter-Sprie GS, Koyama S, Korideck H, et al. Synergy of radiotherapy and PD-1 blockade in Kras-mutant lung cancer. JCI Insight 2016;1:e87415. [Crossref] [PubMed]


