
Lung cancer in women in 21th century
Introduction
Malignant diseases are leading and growing global health problem. The epidemiologic data show an increasing incidence of many cancers. Among them lung cancer is the first cause of death due to malignancy in both sexes. The last 20 years brought spectacular progress in lung cancer diagnosis, recognition of molecular alterations and the mechanisms of host anti-cancer defense leading to treatment strategies with effective tailored therapies. Precise characterization of cancer (i.e., histology, molecular changes, tumor spread = stage of the disease) and patient (i.e., health conditions, performance status) are the basis for proper qualification to therapy.
The main lung cancer types include small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) (currently occurring in more than 85% of cases). The recognition of main NSCLC types became possible thanks to use of immunohistochemistry with antibodies anti p40 and anti TTF1. These are: squamous cell carcinoma (SQCC), adenocarcinoma (ADC), not otherwise specified (NOS) recognized in small biopsy and large cell carcinoma possible to diagnose in surgical biopsy (1). In the cases of ADC and NOS further molecular diagnosis is indicated for mutations in epidermal growth factor receptor (EGFR) gene and after that for rearrangement of anaplastic lymphoma kinase/c-ros oncogene 1 (ALK/ROS1). This molecular characteristic is recently extended with molecular aberrations of BRAF, KRAS, MET, ERBB2, RET (2).
The stage of disease, which is the most important prognostic factor is defined by TNM classification. This system was introduced in the 1940s. The last 8th lung cancer classification has been used since 2016 (3). In practice the application of this TNM system would not be possible without modern methods of imaging, endoscopy and biopsy techniques. Particularly the use of endobronchial ultrasound guided biopsy (endobronchial ultrasound, esophageal ultrasound with biopsy: EBUS/TBNA, EUS/TNA) has considerably improved diagnosis of lymph nodes involvement with cancer cells. The resection of the tumor and radiotherapy remain the methods of choice of radical treatment. But yet, the majority of cases (more than 70%) are in the advanced stages of disease at recognition. The prolonged survival in these cases may be achieved thanks to the application of targeted therapy with tyrosine kinase inhibitors (TKIs) or immunotherapy with immune check points inhibitors (ICIs) (4-6). The results of currently published clinical studies confirm the efficacy of ICIs combined with chemotherapy in advanced NSCLC (6).
The course of lung cancer even among the same histological types, clinical stages and similar molecular pattern is very different and unpredictable. The prognostic and predictive factors for lung cancer are everlastingly investigated. Clinical observation and epidemiological data justify our special attention paid to the distinctiveness of lung cancer in women. The aim of this review is to summarize available reports concerning selected important aspects of female lung cancer.
Taking into account the proper designation of the two terms: ‘sex’ and ‘gender’ we would like to point out that they should be differentiated in scientific elaborations. Biological sex refers to the anatomy and physiology of the reproductive system with secondary sex characteristics, while gender reflects an individual identification of the person. Therefore, the term ‘sex’ is accurate in the context of cancer biology, but the term ‘gender’ is not without significance in the psychological and behavioral context of the disease.
Lung cancer in women over the years
Chest tumors have been known since the 18th century, first mentioned by Batista Morgagni. The first comprehensive description of tumors of the lung was presented by Isaak Adler about one hundred years ago in the monograph Primary malignant growth of the lungs and bronchi: a pathological and clinical study (New York: Longmans, Green & Company 1912) (7,8). The author presented 374 cases of lung cancer estimating that lung cancer cannot be a problem compared to tuberculosis. It accounted for 1% of all malignancies. Unfortunately, no information about sex distribution in these firstly described cases is available. However, a few descriptions of abnormal “nests of epithelial cells…and epithelial clusters in the alveoli upon microscopic examination.” in young woman were cited by Adler to emphasized the role of careful autopsy examination for lung cancer diagnosis (8).
Nowadays the epidemiological data clearly illustrate a real problem of lung cancer. However, they require proper presentation to understand their value. The methodological aspects of the studies should be taken into account when epidemiological data are compared. Some of the cases may be disregarded because of imperfection of the diagnostic procedures or inaccuracy of reporting methods (9). The increasing incidence of the disease may be somewhat relative when diagnosis the is improved and the problem is carefully observed. Consequently, the presented data are not always comparable and following the epidemiological changes over time requires caution. Here we demonstrate incidence and mortality due to lung cancer as a proportion of all malignant tumors (Figure 1). In our opinion it is proper way of showing current epidemiological status and may prove useful in comparable analyses. These pie charts show a dominant place of lung cancer among all malignant tumors. It seems to be reducing from 2011, when its incidence among women was shown to be 14% and mortality—26% of all cancers (12).
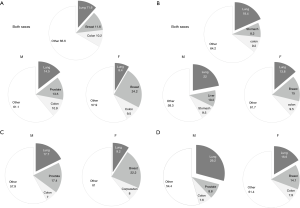
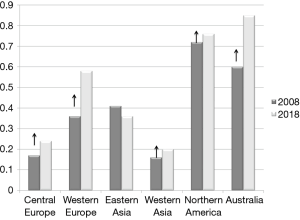
A prolonged survival in the aging population and significant improvement of care of heart and chronic diseases have led to the conditions in which malignant diseases have become a great and serious health problem worldwide. Lung cancer constitute one of the most common cancers among both sexes. Basing on the data from International Agency for Research on Cancer (WHO) the number of new lung cancer cases in 2018 in both sexes was about 2 million, which accounted for 11.6% of all cancers. The estimated global deaths were 1.8 million (1.2 million of males and about 600 thousand of females) what is accounted for 18.4% of all cancer deaths. The lung cancer incidence is the highest in Asia (58.5% of all lung cancer cases), Europe (22.4%) and North America (12.1%) (10). The world averaged incidence age standardized rates are estimated at 31.5 in males and 14.6 in females (per 100,000). The highest age standardized incidence rates in males (more than 40 per 100,000) are reported in Micronesia, Polynesia Central and Eastern Europe and in Eastern and Western Asia. In the countries of North and South America only Uruguay is recorded in the same group. Among women the highest incidence (>24/100,000) was noted in Micronesia, Western and Northern Europe, Northern America and Australia/New Zeeland. In Eastern Europe the age standardized incidence rate was 11.9/100,000 (10). In Poland about 21,000 people received annually a new diagnosis of lung cancer (14,460 men, 7,500 women, according to the data from 2015) (11). Over the last years it was the death cause of about 30% of all malignant neoplasms of males and 15% of females. For about 25 years a downward trend in incidence of lung cancer in males and a tendency to increase in females (Figure 2) have been observed. In the study presented by Radzikowska et al. in 1995 of 5,404 lung cancer patients only 14.5% were women. The mean age of women and men was similar (59.7 vs. 61.9 years, respectively). There was a prevalence of women in the group younger than 50 years with the ratio of male to female 1:3, while in patients over 50 years the ratio was 1:7. The study indicated that 75% of women with lung cancer were smokers (13). In addition, it presented a different profile of women and men with lung cancer in a large group. Jemal et al. analyzed age specific incidence of lung cancer in the US (14). The decreasing incidence of this cancer in both sexes was confirmed but, the decline was greater among men. The results show that among young white non-Hispanic population, female-to-male incidence rate ratio increased from 0.88 in the period 1995–1999 to 1.17 in the period 2010–2014. This ratio was recently the highest among young women diagnosed with localized, less advanced curable disease (15). The authors concluded that this tendency was not fully explained by susceptibility to smoking. A deep analysis of the epidemiology of lung cancer in women in US was presented by Henley et al. (16). Some points are worthy to underline: it was stated that lung cancer incidence does not differ between women and men in the young group of patients. There is a highest incidence of lung cancer among white women. The non-Hispanic women suffer from more aggressive tumors than Hispanic. On the other hand, the survival rate is lower in black women (16).
Smoking
Cigarette smoking peaked in women 2 decades later than in men therefore the epidemic of lung cancer started later in women (17). Women began smoking predominantly during and after World War II. In contrast to men, who began smoking cigarettes at the beginning of the 20th century, with significant peaks of initiation during the two World Wars (18). Nowadays (data from 2016) it is estimated that in the United States 15.5% of all adults (37.8 million) were current cigarette smokers (among them 17.5% of males, 13.5% of females) (19). In Poland smoking habit among women remains high; in 2012, 24.4% of adults smokers were reported to be women (20). The studies by Jassem et al. indicated reduction in the proportion of smokers from 1979 through 2000 to 2013: among men 62.2% by 45% to 34% and among women from 30.3% by 24% to 20% (21,22). However, caution is required when the data concerning smoking are analyzed as some relevant information may be missed due to its collection based on self-reporting.
The susceptibility to harmful influence of tobacco smoke in women is reported with conflicting options. Epidemiological studies show that lung cancer in women is much less tobacco-dependent than in men. It was assessed in 2000 that 47% of lung cancer cases in women were a consequence of tobacco smoke (vs. 85% in men) (23). The group of S. Lam from Canada conducted the investigations of precancerous changes in bronchial epithelium evaluated using fluorescence bronchoscopy and special methods of bronchial biopsy examination (24). In one study 401 subjects were investigated to present sex-related differences in the grading of preinvasive bronchial lesions. The comparable number of cigarette smoking men and women were included in this study. The prevalence of high-grade preinvasive lesions and carcinoma in situ related to smoking were found to be lower in women than in men.
Although some authors reported higher risk of lung cancer development in men, the others did not confirm this observation. O’Keeffe et al. presented a systematic review and meta-analysis of prospective cohort studies (25). In this analysis data from 99 cohorts, of 7,113,303 individuals (46% women) were available. Current smoking was found to be associated with an age-adjusted relative risk (RR) of lung cancer of 7.48 in women and 8.78 in men in comparison to non-smokers. The risk was similarly correlated with the number of cigarettes smoked per day in both sexes. The risk among former smokers did not differ between men and women. The authors concluded that a risk of lung cancer related to smoking was similar in both sexes, however, pointing out that it may remain underestimated as self-reported.
In a large study on 1,300,000 UK women the relationship between smoking with and mortality was analyzed (26). The 12 years relative risk of death of ever smoking vs. never smoking women was found to be second after chronic lung diseases and was 21.4 (19.7–23.2). An important benefit from early smoking cessation was shown in this study. The opinion that women find it more difficult to quitting smoking than men was repeated (27). In our unpublished observation women remained in abstinence longer than men and they were more likely to undergo anti-nicotine therapy.
Taking into account other risk factors for lung cancer development the environmental tobacco smoke (ETS) is not to overestimated. Yet, it is doubtful whether ETS should be included next to other risk factors apart from smoking. Indeed, this agent is extremely difficult to assess and measure. No data concerning ETS in patients anamneses are available, no objective methods of counting ETS “intensity” have been developed (28). However, our knowledge of relationships at home and in the workplace in the near history justifies the decision to estimate a considerable exposure of women to ETS. In Poland more than 60% of men were smokers in the 1970s and 1980s. Today female lung cancer patients aged 70–80 years, who might have spent almost all their lives in the conditions of tobacco smoke exposure are the ‘victims’ of common smoking burden. The mechanisms of action of direct and second-hand tobacco smoke differ. It was presented that side stream of smoke is much more dangerous than the main one. Therefore, ETS is an important agent responsible for such high incidence of lung cancer among “never smoking” women in 21st century.
Lung cancer screening
The only method to achieve an early lung cancer diagnosis in population seems to be a screening with low dose computed tomography (LDCT). The goal of lung cancer screening is to detect tumor in early stage when radical therapy is possible, the survival rate and a reduction of mortality can be achieved. The results of large randomized trial known as the National Lung Screening Trial (NLST) were published in 2011 showing 20% reduction of lung cancer mortality in the LDCT group when compared with classic Chest X-ray or no intervention (29). The debate on the system of qualification to screening program, proper algorithms of care, cost-effectiveness, psychological aspects of lung tumor recognition is ongoing. In recently launched programs one of the criteria is more than 30 pack years smoking history (in majority) (30). It may be discriminating for women, among whom heavy smokers are less numerous than among men. Moreover, lung cancer is a frequent cause of death in never smokers, who are out of screening programs and among whom women are in majority. Interestingly, in NLST the number of women was 10,952 vs. 15,770 of men (29). In the other screening programs a proportion of women was lower: mean 60% for men to 30% for women. In the results of the NELSON study the data concerning sex were available (31). In this study inclusion criteria were as follow: subjects aged 50 to 75 years, smokers with history of more than 15 pack years or ex-smokers from less than 10 years ago. There were 16.5% of women in the study group, 17% of women in the group with lung cancers identified by LDCT. The group of women in this study was younger, with shorter history of smoking and the female patients were classified to significantly lower lung cancer stages than male. All of these results provide the evidence of the rationale for the modification of screening programs which would include women with short smoking history or even the never smoking ones with documented exposure to ETS (16).
There is a prevalence of ADC in women. This type of lung cancer presents different forms with different degree of aggressiveness. The early forms of ADC could be detected as ground glass opacities (GGO) by high-resolution computed tomography (HRCT). The presence of solid part in GGO indicates malignant component and influences on classification and prognosis. The following forms of GGO with solid part are presented in 8th lung cancer classification: cT1mi (minimal invasive ADC) with solid part 0<5 mm; cT1a with solid part 6–10 mm; cT1b with solid part 11–20 mm; and cT1c with solid part 21–30 mm (3). Detection of these early forms of cancer is extremely important giving a chance for radical treatment. This may concern in majority women without smoking history. The course of these forms of lung cancer are mainly asymptomatic with incidental recognition. Unfortunately, this early ADC in nonsmoking women is out of screening range.
Lung cancer and never smoking women
Lung cancer in never-smokers is estimated at 10–15% of all cases and is ranked 7th among all causes of cancer deaths (32). NSCLC among never-smokers is diagnosed in about 53% of women and only 15% of men. Incidence rate of lung cancer among never smokers (per 100,000 person-years) ranged from 14.4 to 20.8 in women and 4.8 to 13.7 in men and ADC is the most commonly diagnosed histological type (33). In Japanese population 75% of female patients with lung cancer are non-smokers (34). Thus, lung cancer in never smokers is a real problem among women. As it was presented in the meta-analysis by O’Keeffe et al., among 500,000 subjects the incidence of lung cancer was 30% higher in never smoking women than men in the US, while in an Australian cohort the proportion of patients with lung cancer was 18% for women and 3% for men (25). Other risk factors apart from smoking are numerous, difficult to assess and are not included in screening programs (30,32). These are radon, indoor fumes from cooking and heating stoves. Lung cancer risk connected with combustion of fuel emission was pointed and found to have high prevalence in women. Many analyses from developing countries confirmed increased risk for lung cancer in women exposed to biomass fuel and cooking. The combustion products contain probable carcinogens (as dibenz/a,h/anthracene, cyclopenta/cd/pyrene and 1,3-butadiene) (35). It is estimated that the lung cancer deaths were connected with PM 2.5 in 10% of men and 18% in women in China. Air pollution is responsible for the significant increase of ADC incidence in China (36). The importance of coal use was emphasized and the association persisted regardless of smoking (37). Recently the additional risk factors for lung cancer e.g., chronic obstructive pulmonary disease (COPD) and fibrotic lung disorders have been identified (38). COPD is not an uniform disease and the emphysema phenotype is much more associated with lung cancer than the bronchitis pattern (39). A risk of lung cancer in COPD patients is reported to be 2- to 6-fold higher regardless of smoking and in many observational studies COPD occurred in about 50% of lung cancer patients. The study of 2,222 patients from China showed 32.6% prevalence of COPD in newly diagnosed lung cancer cases. There were 552 (24.8%) women in this study group, and in 88 women (12.2%) COPD was diagnosed (40). Women seem to be more susceptible to COPD than men although different risk factors to develop this disease in both sexes are known. Women suffering from COPD are younger, with shorter smoking history than men and are likely to have a phenotype with bronchitis rather than emphysema. The decline in the results of pulmonary function tests in post-menopausal women regardless of age related changes was observed (41). The quality of life was lower in women suffering from COPD than in men and less successful long term smoking cessation among women was reported (42).
Interstitial lung diseases with most sever e.g. idiopathic pulmonary fibrosis are recognized more frequently among men with an increased risk of SQCC (38;43). This clinical problem in women needs further investigations.
Intrinsic-individual predisposition
The female susceptibility to carcinogenic effect of tobacco smoke being a subject of different opinion might be explained by genetic predisposition. The clearance of nicotine was found to be higher in women than in men independently of hormonal status (44). The expression of CYP1A1 gene involved in metabolism of polycystic hydrocarbons from tobacco smoke was found to be elevated in women. The glutathione S transferase M1 enzyme mutation, leading to a high probability of lung cancer development in female smokers was also observed (45). In general, the most frequently mutated gene in all types of lung cancer is tumor protein 53 (TP53). A higher incidence of TP53 mutations in women than in men in the group of smokers was observed whereas in non-smokers the results were opposite (46). The lower capacity of DNA repair is observed in women: both smokers and non-smokers, which shows that this genetic alteration plays smoking-independent role in lung cancer (2,47). Gastrin releasing peptide (GRP) is involved in carcinogenesis. The gene for GRP receptor is linked with X chromosome and is expressed in the airway cells of nonsmoking women but not in men (34). ADC is most the frequently recognized histological type of NSCLC in both sexes with significant predominance in women. The reported proportion varies from 40% to 60%. ADC is not a uniform type of cancer with recognizable early lesions (such as atypical adenomatous hyperplasia) visible in GGO on CT up to very aggressive undifferentiated mucinous forms (2,48). In ADC many driver mutations were identified, as follows: EGFR (10−30%), FGFR1 (20%), KRAS (15−30%), PIK3CA (2−5%), ERBB2 (HER2) (2−5%), BRAF (1−3%), ALK (3%), ROS1 (1%) (49). The high rate of these mutations was found in never smokers and in women, and the higher rate was noted in Asia and South America. This group of patients have benefit from targeted therapy which refers to monoclonal antibodies or small molecules TKIs. Data from Asia sheds light on genetics of female lung cancer, which shows certain differences when compared to Caucasian patients. These are: a longer survival, higher response rates to chemotherapy and targeted therapy in Asian patients (50). More than 30% of patients with lung cancer in Asia never had a smoking status and more than 50% of lung cancers occur in women who were never-smokers (51). Meta-analyses (52) which were conducted to characterize the patterns of mutations in NSCLC and comparing them between Western and Asian population demonstrate the difference between the incidence of mutation in connection with ethnic background. EGFR mutation occurred in Asian population in 47.9% of ADC cases while in Western population in 19.2%. Frequency of EGFR mutations in lung cancer is the highest in Vietnam (64.2%), Taiwan (62.1%) and Thailand (53.8%). Also, in the Philippines or China it is observed in more than 50% of patients. It is known that EGFR mutation occurs independently of smoking (53). Driver mutations were detected in 79% of female never-smoker Asian patients with lung ADC and the most frequent was EGFR mutation (63%) (54), whereas KRAS and LKB1 mutations are more frequent in Western population. Soh et al. reported that mutations or copy number gains (CNGs) of the EGFR and KRAS genes differ between Asian and non-Asian patients with lung ADC (55). EGFR CNGs (gEGFR) occurred more frequently than EGFR mutations (mEGFR) (46% vs. 38% in Asians; 21% vs. 10% in non-Asians), whereas KRAS mutations (mKRAS) were more frequent than KRAS CNGs (gKRAS) (13% vs. 7% and 35% vs. 4%, respectively). The never smoking status was associated with mEGFR and gEGFR, in contrast to mKRAS occurred significantly more frequently among patients with smoking history (55). As evidenced by the study in which never-smoking women of East Asian origin with ADC were enrolled, patients with EGFR amplification had a significantly worse outcome in univariate analysis (56). On the other hand in a retrospective population-based study of NSCLC cases between 1991 to 2005. Asian ethnicity was evidenced as an independent favorable prognostic factor for overall survival in NSCLC regardless of smoking status (57).
Hormonal status
Looking for the reason for the specificity of lung cancer biology and clinics in women, the conception of sex dimorphism is taken into consideration. Estrogens are postulated as an important agent in cancer development and progression. At the background—two types of estrogen receptors: ERα and ERβ in the lung epithelium are identified. However, the expression of ERβ is twice more higher than ERα (58) (Figure 3). Their role is to form complete alveolar units in females and males (59). As it was shown in the studies on mice, ERα is responsible for proper differentiation of the lung and provides correct numbers of alveoli per surface, whereas ERβ controls the development of extracellular matrix and provide elasticity (60,61). The expression of ERβ is elevated in lung cancer (62) and it is noticed more often in premenopausal women than in postmenopausal women and in men (63). 17-β-estradiol (E2) which is the most potent form of estrogen was analyzed in the studies as a possible agent in carcinogenesis. The results show that high concentration of 17-β-estradiol can have an important impact on the development of lung cancer (64). For example, in premenopausal women higher concentration of estrogen is associated with worse survival, just as in men with advanced NSCLC—higher concentration of 17-β-estradiol is related to worse survival than in those men with lower. Estrogens may influence metabolism of carcinogens in tobacco smoke, e.g., by induction of CYP1A1 gene (34).
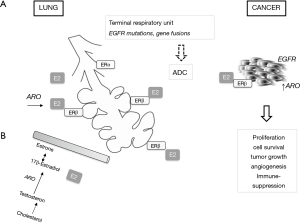
Aromatase (ARO) plays a crucial role in estrogen synthesis. High expression, in even up to 86% of NSCLC tissue in both sexes was found (65,66). The higher ARO expression in metastases than in primary tumors was found suggesting that this enzyme is locally synthetized, which can lead to tumor progression (67). Thus, ARO seems to play an important role in carcinogenesis in lung cancer through the local production of 17-β-estradiol and by conversion of androgens to estrogen also in men (34,68). The data which are capable of confirm that postmenopausal women are at higher risk than premenopausal are limited (69). In one study postmenopausal status with elevated ARO and ERβ expression was predictor to shorted survival (70).
The group of Kucia et al. reported the presence of functional receptors for pituitary sex hormones in lung cancer cell lines (71). They conducted the study to show that pituitary sex hormones are involved in pro-metastatic potency in lung cancer.
There are some reports on the relationship between estrogens and EGFR mutations. It was shown that 17-β-estradiol activates EGFR pathways in NSCLC and the relationship between EGFR and ARO were found. In the study of Deng et al. a high expression of ERβ correlated with EGFR mutations and the opposite prognostic significance was found-positive for EGFR while negative for ERβ expression (72). The use of ARO inhibitors could improve response to TKIs in advanced NSCLC supporting the role of estrogens in EGFR pathways (65,66). Recently an impact of estrogens on the modulation of cancer environment has been evaluated. There is some evidence that E2 signaling contributes to pro-tumor immunity and may have an impact on immunotherapy (73).
All of these results beg the question if hormone replacement therapy (HRT) can contribute to lung cancer development. The answer is still not clear. Some studies have shown an increased risk (even up to 50% for 10 years of use) (74) and higher mortality among women receiving HRT whereas others have not confirmed that association (75,76). The answer to HRT is individual; the prolonged estrogen- progestin usage could contribute to cancer development with genetic predisposition and premalignant epithelial damage (77). Recently some clinical trials with the agents targeted estrogen pathways are conducted. These are: ARO inhibitors, the reversible nonsteroidal agents, ER modulators or degraders (77,78).
Clinical course and treatment
There is growing body of evidence that the outcome in thoracic malignancy in women is better than in men (79,80). Over the years the differences between men and women in the survival rate for many cancers were observed. Up to 37% higher risk of death from cancer in men versus women was reported (80). Some explanation may be simple as in the elderly men suffer from many chronic diseases and these comorbidities significantly worse lung cancer course (COPD, heart failure, atherosclerosis, diabetes). All of the above are at least related to smoking and tobacco smoking is an unfavorable prognostic factor in malignant disease and women are saved in this aspect. The large study by Wainer et al. was performed to compare sex differences in the context of TNM staging (80). In their study two data set were compared: Australian surgical base (555 patients) and American-SEER registry of patients who had underwent resection (47,706 patients). The aim of the study was to assess the effect of sex on survival in each stage accordingly to the new 8th classification. A prevalence of never smokers among women was noted, but there were only in 27.7%; 72.3% were ever smokers. The ADC was significantly higher in women than in men and it was more than 65%. The output proportion of women and men in each NSCLC stage was comparable. However, the 5-year survival for women was significantly higher than for men (in the Australian cohort 68.9% vs. 53.9%, in SEER 65.6% vs. 55.2% respectively). This difference remains unchanged after multivariate analysis including age, smoking history, ECOG PS, histology. These observations lead to the conclusion that “incorporating of sex…would allow for more accurate prognostication and heterogeneity currently seen in TNM staging”.
The response to the newest therapies among female is of interest. As benefits of TKIs in women is well known, the results of immunotherapy with ICIs in women are the subject of research. Our studies we did not find any significant influence of sex on immune status of lung cancer patients (81-83). In the recently published meta-analysis of 23 randomized clinical trials no differences in response to ICIs between sexes were found (84). In the first study showing superiority of nivolumab vs. docetaxel the proportion of women was very low, which was not surprising because the study groups included only patients with SQCC (85). In the Keynote trial with pembrolizumab all lung cancer types were incorporated and proportion of males to females was comparable. The greater benefit of the treatment was found for men. In the study with durvalumab the proportion of women was 30% and the results of treatment were similar in both sexes (86). In the OAK study conducted with atezolizumab proportion of women was 39%. The results showed that the overall survival in the group treated with ICI was longer in women when compared to docetaxel and when compared with men (16.2 vs. 11.2 vs. 12.6 months respectively) (87). In the meta-analysis presented by Pinto et al. no clear benefit for women of nivolumab nor pembrolizumab when compared with chemotherapy was found (88). It may be expected that the influence of sex on ICIs effectiveness will be observed and better elucidated due to the common use of this treatment in practice.
Conclusions
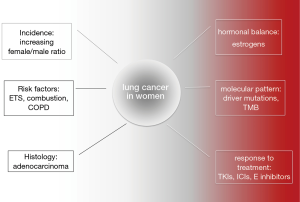
The data presented in this review show that the attention should be focused on the dissimilarity of lung cancer in women. Figure 4 presents the main aspects of lung cancer in women, importance of some risk factors and the main topics for research. There is evidence showing different tendency in the lung cancer rate in both sexes showing slow decrease or even a lack of decrease of the incidence in women. The different risk factors in women should lead to certain modification in screening programs. More effort should be put into the treatment of nicotine addiction in young women. The exploration of other than cigarette smoke environmental risk factors could bring health-related legal regulations. Finally, the prevalence of ADC as ‘female’ lung cancer requires wide area of exploration in the aspect of genetics, female hormone pathways and modification of the tumor environment. There is a growing body of evidence as to how much effort must be put into the reduction of the spread of lung cancer in women.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-287). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Testa U, Castelli G, Pelosi E. Lung Cancers: Molecular Characterization, Clonal Heterogeneity and Evolution, and Cancer Stem Cells. Cancers (Basel) 2018;10:248. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Larsen JE, Cascone T, Gerber DE, et al. Targeted therapies for lung cancer: clinical experience and novel agents. Cancer J 2011;17:512-27. [Crossref] [PubMed]
- Domagała-Kulawik J. Immune checkpoint inhibitors in non-small cell lung cancer - towards daily practice. Adv Respir Med 2018.86. [PubMed]
- Brahmer JR, Govindan R, Anders RA, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of non-small cell lung cancer (NSCLC). J Immunother Cancer 2018;6:75. [Crossref] [PubMed]
- Domagała-Kulawik J. Lung cancer. In 100 years of monograph by Issak Adler. Pneumonol Alergol Pol 2012;80:581-2. [PubMed]
- Classics in oncology. Primary malignant growths of the lung. Isaac A. Adler, A.M., M.D. CA Cancer J Clin 1980;30:295-301. [Crossref] [PubMed]
- Rich A, Baldwin D, Alfageme I, et al. Achieving Thoracic Oncology data collection in Europe: a precursor study in 35 Countries. BMC Cancer 2018;18:1144. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Ditkowska J, Wojciechowska U, Olasek P. Cancer in Poland in 2015. Krajowy Rejestr Nowotworów 2017.
- Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med 2011;32:605-44. [Crossref] [PubMed]
- Radzikowska E, Głaz P. Lung cancer--differences of incidence between the sexes. Pneumonol Alergol Pol 2000;68:417-24. [PubMed]
- Jemal A, Miller KD, Ma J, et al. Higher Lung Cancer Incidence in Young Women Than Young Men in the United States. N Engl J Med 2018;378:1999-2009. [Crossref] [PubMed]
- Jemal A, Ma J, Siegel RL. Incidence of Lung Cancer among Young Women. N Engl J Med 2018;379:990-1. [PubMed]
- Henley SJ, Gallaway S, Singh SD, et al. Lung Cancer Among Women in the United States. J Womens Health (Larchmt) 2018;27:1307-16. [Crossref] [PubMed]
- Alberg AJ, Brock MV, Ford JG, et al. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e1S-29S.
- Jemal A, Thun MJ, Ries LA, et al. Annual report to the nation on the status of cancer, 1975-2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst 2008;100:1672-94. [Crossref] [PubMed]
- Jamal A, King BA, Neff LJ, et al. Current Cigarette Smoking Among Adults - United States, 2005-2015. MMWR Morb Mortal Wkly Rep 2016;65:1205-11. [Crossref] [PubMed]
- Giovino GA, Mirza SA, Samet JM, et al. Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross-sectional household surveys. Lancet 2012;380:668-79. [Crossref] [PubMed]
- Jassem J, Przewozniak K, Zatonski W. Tobacco control in Poland-successes and challenges. Transl Lung Cancer Res 2014;3:280-5. [PubMed]
- Wramner B, Zatonski W, Pellmer K. Premature mortality in lung cancer as an indicator of effectiveness of tobacco use prevention in a gender perspective--a comparison between Poland and Sweden. Cent Eur J Public Health 2001;9:69-73. [PubMed]
- Bray F, Tyczynski JE, Parkin DM. Going up or coming down? The changing phases of the lung cancer epidemic from 1967 to 1999 in the 15 European Union countries. Eur J Cancer 2004;40:96-125. [Crossref] [PubMed]
- Lam S, leRiche JC, Zheng Y, et al. Sex-related differences in bronchial epithelial changes associated with tobacco smoking. J Natl Cancer Inst 1999;91:691-6. [Crossref] [PubMed]
- O'Keeffe LM, Taylor G, Huxley RR, et al. Smoking as a risk factor for lung cancer in women and men: a systematic review and meta-analysis. BMJ Open 2018;8:e021611. [Crossref] [PubMed]
- Pirie K, Peto R, Reeves GK, et al. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet 2013;381:133-41. [Crossref] [PubMed]
- North CM, Christiani DC. Women and lung cancer: what is new? Semin Thorac Cardiovasc Surg 2013;25:87-94. [Crossref] [PubMed]
- Ni X, Xu N, Wang Q. Meta-Analysis and Systematic Review in Environmental Tobacco Smoke Risk of Female Lung Cancer by Research Type. Int J Environ Res Public Health 2018;15:1348. [Crossref] [PubMed]
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Kauczor HU, Bonomo L, Gaga M, et al. ESR/ERS white paper on lung cancer screening. Eur Respir J 2015;46:28-39. [Crossref] [PubMed]
- Horeweg N, van der Aalst CM, Thunnissen E, et al. Characteristics of lung cancers detected by computer tomography screening in the randomized NELSON trial. Am J Respir Crit Care Med 2013;187:848-54. [Crossref] [PubMed]
- Samet JM, Avila-Tang E, Boffetta P, et al. Lung cancer in never smokers: clinical epidemiology and environmental risk factors. Clin Cancer Res 2009;15:5626-45. [Crossref] [PubMed]
- Wakelee HA, Chang ET, Gomez SL, et al. Lung cancer incidence in never smokers. J Clin Oncol 2007;25:472-8. [Crossref] [PubMed]
- Siegfried JM. Women and lung cancer: does oestrogen play a role? Lancet Oncol 2001;2:506-13. [Crossref] [PubMed]
- Kc R, Shukla SD, Gautam SS, et al. The role of environmental exposure to non-cigarette smoke in lung disease. Clin Transl Med 2018;7:39. [Crossref] [PubMed]
- Parascandola M, Xiao L. Tobacco and the lung cancer epidemic in China. Transl Lung Cancer Res 2019;8:S21-30. [Crossref] [PubMed]
- Kurmi OP, Arya PH, Lam KB, et al. Lung cancer risk and solid fuel smoke exposure: a systematic review and meta-analysis. Eur Respir J 2012;40:1228-37. [Crossref] [PubMed]
- Naccache JM, Gibiot Q, Monnet I, et al. Lung cancer and interstitial lung disease: a literature review. J Thorac Dis 2018;10:3829-44. [Crossref] [PubMed]
- Denholm R, Schuz J, Straif K, et al. Is previous respiratory disease a risk factor for lung cancer? Am J Respir Crit Care Med 2014;190:549-59. [Crossref] [PubMed]
- Wang W, Dou S, Dong W, et al. Impact of COPD on prognosis of lung cancer: from a perspective on disease heterogeneity. Int J Chron Obstruct Pulmon Dis 2018;13:3767-76. [Crossref] [PubMed]
- Triebner K, Matulonga B, Johannessen A, et al. Menopause Is Associated with Accelerated Lung Function Decline. Am J Respir Crit Care Med 2017;195:1058-65. [Crossref] [PubMed]
- Jenkins CR, Chapman KR, Donohue JF, et al. Improving the Management of COPD in Women. Chest 2017;151:686-96. [Crossref] [PubMed]
- Tomassetti S, Gurioli C, Ryu JH, et al. The impact of lung cancer on survival of idiopathic pulmonary fibrosis. Chest 2015;147:157-64. [Crossref] [PubMed]
- Hukkanen J, Jacob P III, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev 2005;57:79-115. [Crossref] [PubMed]
- Tang DL, Rundle A, Warburton D, et al. Associations between both genetic and environmental biomarkers and lung cancer: evidence of a greater risk of lung cancer in women smokers. Carcinogenesis 1998;19:1949-53. [Crossref] [PubMed]
- Toyooka S, Tsuda T, Gazdar AF. The TP53 gene, tobacco exposure, and lung cancer. Hum Mutat 2003;21:229-39. [Crossref] [PubMed]
- Kligerman S, White C. Epidemiology of lung cancer in women: risk factors, survival, and screening. AJR Am J Roentgenol 2011;196:287-95. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc 2011;8:381-5. [Crossref] [PubMed]
- Levy MA, Lovly CM, Pao W. Translating genomic information into clinical medicine: lung cancer as a paradigm. Genome Res 2012;22:2101-8. [Crossref] [PubMed]
- Soo RA, Kawaguchi T, Loh M, et al. Differences in outcome and toxicity between Asian and caucasian patients with lung cancer treated with systemic therapy. Future Oncol 2012;8:451-62. [Crossref] [PubMed]
- Toh CK, Wong EH, Lim WT, et al. The impact of smoking status on the behavior and survival outcome of patients with advanced non-small cell lung cancer: a retrospective analysis. Chest 2004;126:1750-6. [Crossref] [PubMed]
- Dearden S, Stevens J, Wu YL, et al. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Ann Oncol 2013;24:2371-6. [Crossref] [PubMed]
- Mitsudomi T. Molecular epidemiology of lung cancer and geographic variations with special reference to EGFR mutations. Transl Lung Cancer Res 2014;3:205-11. [PubMed]
- Ha SY, Choi SJ, Cho JH, et al. Lung cancer in never-smoker Asian females is driven by oncogenic mutations, most often involving EGFR. Oncotarget 2015;6:5465-74. [Crossref] [PubMed]
- Soh J, Toyooka S, Matsuo K, et al. Ethnicity affects EGFR and KRAS gene alterations of lung adenocarcinoma. Oncol Lett 2015;10:1775-82. [Crossref] [PubMed]
- Sholl LM, Yeap BY, Iafrate AJ, et al. Lung adenocarcinoma with EGFR amplification has distinct clinicopathologic and molecular features in never-smokers. Cancer Res 2009;69:8341-8. [Crossref] [PubMed]
- Ou SH, Ziogas A, Zell JA. Asian ethnicity is a favorable prognostic factor for overall survival in non-small cell lung cancer (NSCLC) and is independent of smoking status. J Thorac Oncol 2009;4:1083-93. [Crossref] [PubMed]
- Ivanova MM, Mazhawidza W, Dougherty SM, et al. Activity and intracellular location of estrogen receptors alpha and beta in human bronchial epithelial cells. Mol Cell Endocrinol 2009;305:12-21. [Crossref] [PubMed]
- Tam A, Morrish D, Wadsworth S, et al. The role of female hormones on lung function in chronic lung diseases. BMC Womens Health 2011;11:24. [Crossref] [PubMed]
- Massaro D, Massaro GD. Estrogen regulates pulmonary alveolar formation, loss, and regeneration in mice. Am J Physiol Lung Cell Mol Physiol 2004;287:L1154-9. [Crossref] [PubMed]
- Massaro D, Massaro GD. Estrogen receptor regulation of pulmonary alveolar dimensions: alveolar sexual dimorphism in mice. Am J Physiol Lung Cell Mol Physiol 2006;290:L866-70. [Crossref] [PubMed]
- Siegfried JM, Hershberger PA, Stabile LP. Estrogen receptor signaling in lung cancer. Semin Oncol 2009;36:524-31. [Crossref] [PubMed]
- Rodriguez-Lara V, Pena-Mirabal E, Baez-Saldana R, et al. Estrogen receptor beta and CXCR4/CXCL12 expression: differences by sex and hormonal status in lung adenocarcinoma. Arch Med Res 2014;45:158-69. [Crossref] [PubMed]
- Bai Y, Shen W, Zhu M, et al. Combined detection of estrogen and tumor markers is an important reference factor in the diagnosis and prognosis of lung cancer. J Cell Biochem 2019;120:105-14. [Crossref] [PubMed]
- Rodriguez-Lara V, Hernandez-Martinez JM, Arrieta O. Influence of estrogen in non-small cell lung cancer and its clinical implications. J Thorac Dis 2018;10:482-97. [Crossref] [PubMed]
- Weinberg OK, Marquez-Garban DC, Fishbein MC, et al. Aromatase inhibitors in human lung cancer therapy. Cancer Res 2005;65:11287-91. [Crossref] [PubMed]
- Márquez-Garbán DC, Chen HW, Goodglick L, et al. Targeting aromatase and estrogen signaling in human non-small cell lung cancer. Ann N Y Acad Sci 2009;1155:194-205. [Crossref] [PubMed]
- Niikawa H, Suzuki T, Miki Y, et al. Intratumoral estrogens and estrogen receptors in human non-small cell lung carcinoma. Clin Cancer Res 2008;14:4417-26. [Crossref] [PubMed]
- Min L, Wang F, Liang S, et al. Menopausal status and the risk of lung cancer in women: A PRISMA-compliant meta-analysis. Medicine (Baltimore) 2017;96:e7065. [Crossref] [PubMed]
- Mah V, Seligson DB, Li A, et al. Aromatase expression predicts survival in women with early-stage non small cell lung cancer. Cancer Res 2007;67:10484-90. [Crossref] [PubMed]
- Abdelbaset-Ismail A, Pedziwiatr D, Schneider G, et al. Pituitary sex hormones enhance the prometastatic potential of human lung cancer cells by downregulating the intracellular expression of heme oxygenase1. Int J Oncol 2017;50:317-28. [Crossref] [PubMed]
- Deng F, Li M, Shan WL, et al. Correlation between epidermal growth factor receptor mutations and the expression of estrogen receptor-beta in advanced non-small cell lung cancer. Oncol Lett 2017;13:2359-65. [Crossref] [PubMed]
- Rothenberger NJ, Somasundaram A, Stabile LP. The Role of the Estrogen Pathway in the Tumor Microenvironment. Int J Mol Sci 2018;19:611. [Crossref] [PubMed]
- Slatore CG, Chien JW, Au DH, et al. Lung cancer and hormone replacement therapy: association in the vitamins and lifestyle study. J Clin Oncol 2010;28:1540-6. [Crossref] [PubMed]
- Brinton LA, Schwartz L, Spitz MR, et al. Unopposed estrogen and estrogen plus progestin menopausal hormone therapy and lung cancer risk in the NIH-AARP Diet and Health Study Cohort. Cancer Causes Control 2012;23:487-96. [Crossref] [PubMed]
- Chlebowski RT, Anderson GL, Manson JE, et al. Lung cancer among postmenopausal women treated with estrogen alone in the women's health initiative randomized trial. J Natl Cancer Inst 2010;102:1413-21. [Crossref] [PubMed]
- Hsu LH, Chu NM, Kao SH. Estrogen, Estrogen Receptor and Lung Cancer. Int J Mol Sci 2017;18:1713. [Crossref] [PubMed]
- Almotlak AA, Farooqui M, Siegfried JM. Inhibiting pathways predicted from a steroid hormone gene signature yields synergistic anti-tumor effects in non-small cell lung cancer. J Thorac Oncol 2020;15:62-79. [Crossref] [PubMed]
- Nakamura H, Ando K, Shinmyo T, et al. Female gender is an independent prognostic factor in non-small-cell lung cancer: a meta-analysis. Ann Thorac Cardiovasc Surg 2011;17:469-80. [Crossref] [PubMed]
- Wainer Z, Wright GM, Gough K, et al. Sex-Dependent Staging in Non-Small-Cell Lung Cancer; Analysis of the Effect of Sex Differences in the Eighth Edition of the Tumor, Node, Metastases Staging System. Clin Lung Cancer 2018;19:e933-44.
- Domagała-Kulawik J, Hoser G, Droszcz P, et al. T-cell subtypes in bronchoalveolar lavage fluid and in peripheral blood from patients with primary lung cancer. Diagn Cytopathol 2001;25:208-13. [Crossref] [PubMed]
- Domagała-Kulawik J, Guzman J, Costabel U. Immune cells in bronchoalveolar lavage in peripheral lung cancer--analysis of 140 cases. Respiration 2003;70:43-8. [Crossref] [PubMed]
- Kwiecien I, Stelmaszczyk-Emmel A, Polubiec-Kownacka M, et al. Elevated regulatory T cells, surface and intracellular CTLA-4 expression and interleukin-17 in the lung cancer microenvironment in humans. Cancer Immunol Immunother 2017;66:161-70. [Crossref] [PubMed]
- Wallis CJD, Butaney M, Satkunasivam R, et al. Association of Patient Sex With Efficacy of Immune Checkpoint Inhibitors and Overall Survival in Advanced Cancers: A Systematic Review and Meta-analysis. JAMA Oncol 2019;5:529-36. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Pinto JA, Vallejos CS, Raez LE, et al. Gender and outcomes in non-small cell lung cancer: an old prognostic variable comes back for targeted therapy and immunotherapy? ESMO Open 2018;3:e000344. [Crossref] [PubMed]


