Virtual thoracoscopic imaging-assisted pleural marking of pulmonary nodules
Introduction
In recent years, computed tomography (CT) screening for lung cancer has been able to detect small pulmonary nodules (1). The need for diagnostic resection with video-assisted thoracoscopic surgery (VATS) is increasing (2). However, finger palpation of nodules via a small access port is ambiguous. The conversion rate for tumor localization has been reported between 13% and 50%, and tumor markings are considered useful for reducing the conversion rate (3,4). Previously reported methods of localizing small pulmonary nodules have included preoperative bronchoscopy or preoperative puncture and dye injection (5,6). However, preoperative bronchoscopy is traumatic for patients, and piercing the chest wall is a maneuver with risk of a vascular injury.
We have previously reported a method of preoperative cutaneous marking and intraoperative pleural marking for localizing pulmonary peripheral nodules (7). In this method, although preoperative CT-guided cutaneous marking with permanent pen was necessary, intraoperative piercing of the chest wall to facilitate pleural marking did not cause pain for patients. However, the required preoperative preparation, a few days before surgery, was complicated.
We have improved the method of applying a marker with dye to the parietal pleura, just above the tumor, using a virtual thoracoscopic image reconstructed from chest CT images. This is followed by two-lung ventilation in which the dye is transferred to the visceral pleura just above the tumor. This study aimed to evaluate the accuracy and safety of this new marking technique.
We present the following article per the STROBE guideline checklist (available at
Methods
The present study was approved by the Institutional Review Board of Kanazawa Medical University Hospital (I405), and the study was registered with UMIN Clinical Trials Registry in Japan (UMIN-CTR ID: UMIN000037661). The study was performed in accordance with the Helsinki Declaration as revised in 2013. Individual informed consent was obtained from each patient.
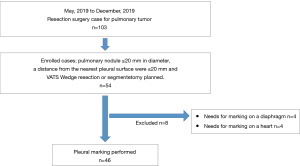
The inclusion criteria were as follows: a peripheral pulmonary nodule ≤20 mm in diameter, a distance from the nearest pleural surface of ≤20 mm, a planned VATS wedge resection or segmentectomy at Kanazawa Medical University Hospital, and patients aged more than 20 years. Patients with no definitive diagnosis before surgery and who needed frozen section diagnosis by VATS wedge resection to perform the VATS lobectomy were included. However, the cases requiring marker on the diaphragm or heart were excluded. The cases that needed putting on a marker on 46 patients were enrolled between May and December 2019.
Technique
Before surgery, the location of the target pulmonary nodule was identified on preoperative chest CT by the costal number and distance from a designated landmark (rib head or edge of parietal pleura) (Figure 1A). A virtual thoracoscopic image was made using the preoperative CT image and Ziostation2 volume rendering software (Ziosoft Inc., Tokyo) (Figure 1B). The method of creating the virtual thoracoscopic image was as follows. 3D reconstruction was performed from the preoperative CT image. The caudal object from the diaphragm was subtracted. At this point, a similar view to that of a thoracoscope was obtained by looking at this object from the caudal side.
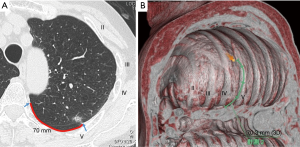
The location of the tumor was confirmed on the axial image, and the chest wall region closest to this was marked on the 3D image. However, since the pulmonary vascular were also synthesized in this image, by selecting the pulmonary blood vessels and subtracting them from the 3D reconstructed image, an image closer to the chest wall image observed by the thoracoscope can be obtained.
The surgical approach was as follows. The operator’s approach port was skin incision 4cm in size on the 4th inter costal space on the anterior axillary line. The camera port was a 2 cm skin incision in the 7th inter costal space on the middle axillary line. And the assistant’s port was a 2 cm skin incision in the 7th intercostal space of caudal side of a scapula. Under the thoracoscopic view, during thoracoscopic surgery, the virtual thoracoscopic image was used as a reference to identify the pleural point on the expected rib, and the distance from the designated landmark was measured using a paper ruler from the operator’s port (Figure 2A). The pleural marker was made of surgical tape, cut into 1 cm pieces and mixed with dye (Pyoktanin Blue® 1% water solution; Wako Pure Chemical Industries Ltd., Osaka, Japan) and sterilized gel for ultrasonography (Sterlite Aquasonic 100®; Parker Laboratories Inc., Fairfield, NJ). The mixed dye was prepared by mixing 0.5 mL of dye and 1.5 mL gel. The prepared marker with mixed dye was then attached to the identified pleural point from the operator’s port using forceps (Figure 2B).
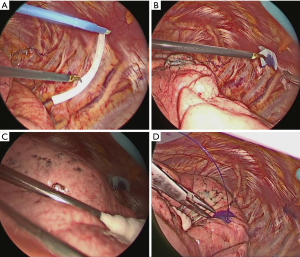
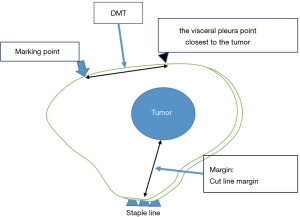
After attaching the marker to the parietal pleura, two-lung ventilation was performed at a pressure of 20 cmH2O for 10 seconds to expand the lungs (Figure 2C). To avoid marker dragging by expanding the lung, we have squeezed until the lungs were completely expanded. One-lung ventilation was performed again on the marked side to confirm that the pigment had adhered to the lung surface. Subsequently, the pleural marker was removed (Figure 2D). In order to accurately measure the distance between the marking point and the pleura closest to the tumor, a 3-0 Polydioxanone (PDS) yarn was pierced and ligated at the center of the pigmented marking, and finger palpation was performed (Figure 3). The technical aspects for each step are shown in Video 1.
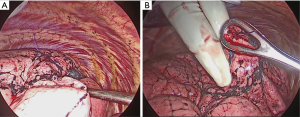
A wedge resection of the palpable tumor was performed. For impalpable tumors, the surgeon was able to perform wider wedge resection or anatomical pulmonary resection. The presence of tumors was confirmed in all resected lungs by intra-operative frozen section diagnosis. To evaluate the accuracy of the marking, pathologists measured the distance between the center of the marking and the visceral pleura closest to the tumor (DMT) before creating the frozen sections (Figure 4).
Statistical analysis
The primary endpoint was DMT, and the secondary endpoint was the cut line margin (margin). The Easy R software program (Saitama Medical Center, Jichi Medical University, Saitama, Japan) was used for the analysis.
Results
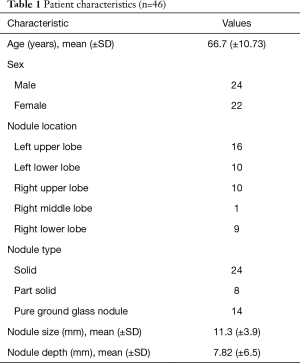
A total of 46 patients were enrolled between May and October 2019 (Figure 5). The patient characteristics are listed in Table 1. The mean nodule size was 11.3 mm. The mean nodule depth below the visceral pleura was 7.82 mm. No complications related to the procedure were observed during or after any of the surgeries.
Full table
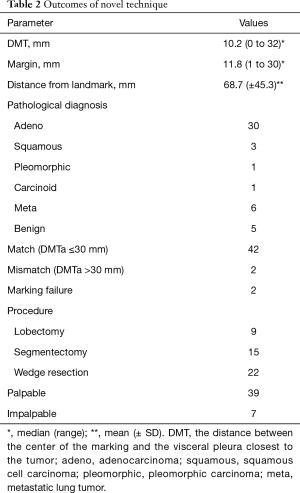
The mean DMT was 10.2 mm (range, 0–32 mm), and the mean margin was 11.8 mm (range, 2–30 mm) (Table 2). The average distance measured by a ruler was 68.7 mm. In two cases, the marker could not be placed because of positional difficulties near the pleural apex and the dorsal side of the diaphragm.
Full table
All tumors were fully resected. In 42/46 cases (91.3%), the DMT was within 30 mm of the marking. Surgical procedures included 22 wedge resections, 15 segmentectomies, and 9 lobectomies; 13 segmentectomies were performed after wedge resection, and seven lobectomies were performed after wedge resection.
The palpable tumor rate was 39/46 (84.8%). The unpalpable tumors were resected using wider partial resection in two cases, segmentectomy in two cases, and lobectomy in two cases. No case required conversion to open surgery for palpation of the tumor. In the wider wedge resection case, the operator had incorrectly identified the point of the targeted tumor on palpation; however, the tumor was resected with the marking yarn because it was proximal to the palpated tumor.
Discussion
Preoperative marking methods for the small pulmonary nodules are mainly divided into transbronchial marking using bronchoscopy and CT-guided percutaneous marking. Transbronchial marking is an invasive, complicated, and time-consuming procedure that requires bronchoscopy before surgery, and the virtual-assisted lung mapping (VAL-MAP) procedure described by Sato et al. requires volume rendering software to create a virtual bronchoscopy image (5). On the other hand, preoperative percutaneous markings used hook wire and pigment (8,9). Complications, such as pneumothorax, hemothorax, hemorrhage, or air embolism, due to puncturing of the lung parenchyma are well known. For this reason, preoperative CT-guided marking of the body surface, without any risk of lung puncture, has also been reported (10-12).
These reports describe the process of marking the pleura using a cotton ball or catheter with dye fixed via needle puncture during surgery. The devices were inserted percutaneously into the pleural cavity guided by a preoperative cutaneous marking. This method avoids puncturing the lung parenchyma and complications including an air embolism, hemorrhage, or pneumothorax. However, this method is not feasible in tumors near the mediastinum, heart, or major vessels like the aorta. In addition, the chest wall cannot be pierced when the tumor is behind the scapula.
Our previously described technique used for localizing pulmonary nodules required a preoperative CT image for cutaneous marking and needle puncture for pleural marking (7). The needle puncture indicated the point of the parietal pleura just above the tumor. Tumors located above the scapula or near the mediastinum were marked by inserting two needles at equal distances from the tumor and placing a pleural marker at the midpoint. This indirect marking method stimulate the development of the method described in the present study.
In the pleural cavity, a specific point on the chest wall can be identified using a coordinate system based on the number of the associated rib and the distance from a landmark such as a the mediastinum pleural edge or a rib head. The merit of the present method is that it does not require piercing of the chest wall. This non-puncturing method enables safe marking even for tumors near the heart, major vessel, or mediastinum. It is also beneficial when used for apical tumors as it avoids the risk of erroneous marker placement that can occur due to deviations in piercing angle due to chest wall thickness.
Using virtual thoracoscopic images, we can avoid the incorrect determination of the coordinate system and rib number. Even if there are no virtual images, it may be possible to utilize the coordinate system by locating the costal number and landmark point on horizontal CT images. Some surgeons affixed pleural markers without viewing the virtual images during surgery because they memorized the coordinates of the rib number and the distance from the landmark. However, if they were not confident about the rib number, as viewed via the thoracoscopic monitor image, they referred to the virtual thoracoscopic image. Since the first or second caput costae is hidden by the chest wall muscles or subpleural fat, the virtual thoracoscopic image can aid in accurately counting the number of ribs.
Some nodules appear easily accessible on preoperative CT images but are actually very difficult to palpate. In other cases, the tumor is expected to be difficult to palpate or the surgeon may wonder if the palpated nodule is really the targeted one. In these situations, if the tumor is located in the peripheral lung and is not in contact with the diaphragm or heart, this virtual imaging method can be used to assist the surgeon with palpation and facilitate excision of the tumor.
Regarding the accuracy of the marking, in the previously reported method of preoperative cutaneous marking and intraoperative pleural marking, the mean DMT was 12.3 mm (7). In the present method, the mean DMT was 10.2 mm, without a lateral CT taken before surgery. Okuda et al. reported a method similar to the present method (13). They did not mark the surface of the body but fixed a dye-coated cotton-tipped stick to a site that appeared anatomically close to the tumor and marked the visceral pleura near the tumor. The mean DMT was 18.2 mm larger than that in our method. The cotton sticks with dye that were used might be the cause of this larger variation. On the other hand, Mun et al. reported a mean distance from the nodule to the marked point of 7.0 mm (range, 0–30 mm) (12). In their method, pleural marking was performed using an epidural catheter and dye. The catheter was fixed onto the chest wall. However, in the present method, the pleural marker was not fixed onto the parietal pleura and may have been easily dragged by the expanding lungs. This dragging may be one reason for discrepancies between the pleural marking site and the location of the tumor. In addition, the relaxed diaphragm may inhibit lung expansion and contribute to the deviation. We utilized virtual thoracoscopic images, reconstructed from the preoperative CT images taken on inhalation. However, the diaphragm was relaxed intraoperatively.
In this study, there were two cases in which the marker could not be placed on the target chest wall point. Fortunately, the finger palpation without marking was able to detect the nodule, in these cases. In case 15, placement was limited by the position of the access port. The movable angle of the forceps was restricted in the intercostal space, creating a region where the marker could not be affixed. In such a situation, application from a different access port might have been effective. In case 10, the target point on the chest wall was behind the diaphragm and could not be seen by thoracoscopy without synchronous exclusion of the lung and diaphragm with forceps. Therefore, the marker could not be placed due to technical difficulty. Therefore, after case 10, we have caudally excluded the diaphragm during marking and transferred dye to the lung surface by bilateral ventilation. This enabled marking on the lung surface even on the dorsal side of the diaphragm. In addition to this, preoperative CT was performed in a supine position, and we have made the virtual thoracoscopic image using this image. However, all patients are positioned in lateral position during surgery. Initially there was concern about the location of the tumor in the supine and lateral positions. In the previous study, we marked the body surface under CT guide in the lateral position before the surgery (7). However, in this study, since the previous study and DMT were almost unchanged, it was considered that even the CT imaged in the supine position had the same accuracy as under the marking in the lateral position.
We used a sterile echogenic jelly mixed with dye, placed on a sterilized surgical tape, as a pleural marker as this prevents the parietal pleura from coming into direct contact with the jelly and dye. The echogenic jelly was suitable for solutes of liquid pigments, and the mixture of pigments and gel was useful for keeping the pleural surface of the lungs. Since the liquid dye easily spreads widely from the lung surface, the target area is easily blurred. Therefore, mixed gel was suitable for pleural marking. A sterilized echogenic jelly is said not to cause tissue degeneration during subcutaneous insertion (14) and is guaranteed to be safe as a material used for marking. Additionally, shielding by the surgical tape may make it safer.
The limitation of this technique is that palpation of the tumor remains indispensable. Position misalignment between the marking and the tumor is on average about 10.2 mm; however, this is still quite close to the tumor. In two cases (4.3%), the site of the actual tumor differed greatly from the marking site. Therefore, our technique cannot guarantee that the marking and the tumor will line up perfectly. If we can reduce the likelihood of the pleural marker being dragged, the marking accuracy can be improved. Other marker materials, which can better adhere to the pleura, may reduce the dragging that occurs.
Furthermore, in the present study, we have excluded the cases that needed putting the marker on the heart or the diaphragm because doing so has a risk of arrhythmia and injury, and it is difficult to put the marker on the correct point with the rib coordinate system. However, even with the nodule near to the heart or major vessels, putting the pleural marker onto the chest wall was possible but we have avoided to put the marker on the heart or diaphragm. For the cases in which putting the marker on the heart or diaphragm cannot be avoided, the present method is difficult to perform.
Conclusions
Thoracoscopic pleural marking for peripheral pulmonary nodules may aid in palpating the tumor and confirming the cut line. The only preoperative requirement for our method is a thoracoscopic image reconstructed from a CT image. The procedure is easy and safe and requires no highly technical devices.
Acknowledgments
The authors would like to thank S. Yamada, M. Kumagai, and K. Mizutani for measuring the outcomes in this study. We also thank T. Nagata for reconstructing the virtual thoracoscopic images.
Funding: This work was supported in part by Pfizer Co., Ltd., Shionogi Pharmaceutical Co., Ltd., Boehringer Ingelheim Japan Co., Ltd., Chugai Pharmaceutical Co., Ltd. Daiichi Sankyo Co., Ltd., and Taiho Pharmaceutical Co., Ltd. The authors had full control of the design of the study, methods used, outcome parameters, analysis of data, and production of the written report. All authors declare freedom of investigation for this work.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-805
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-805
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-20-805
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-805). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The present study was approved by the Institutional Review Board of Kanazawa Medical University Hospital (I405), and the study was registered with UMIN Clinical Trials Registry in Japan (UMIN-CTR ID: UMIN000037661). The study was performed in accordance with the Helsinki Declaration as revised in 2013. Individual informed consent was obtained from each patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Saghir Z, Dirksen A, Ashraf H, et al. CT screening for lung cancer brings forward early disease. The randomised Danish Lung Cancer Screening Trial: status after five annual screening rounds with low-dose CT. Thorax 2012;67:296-301. [Crossref] [PubMed]
- Mack MJ, Hazelrigg SR, Landreneau RJ, et al. Thoracoscopy for the diagnosis of the indeterminate solitary pulmonary nodule. Ann Thorac Surg 1993;56:825-30; discussion 830-2. [Crossref] [PubMed]
- Cardillo G, Regal M, Sera F, et al. Videothoracoscopic management of the solitary pulmonary nodule: a single-institution study on 429 cases. Ann Thorac Surg 2003;75:1607-11. [Crossref] [PubMed]
- Suzuki K, Nagai K, Yoshida J, et al. Video-Assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest 1999;115:563-8. [Crossref] [PubMed]
- Sato M, Yamada T, Menju T, et al. Virtual-assisted lung mapping: outcome of 100 consecutive cases in a single institute. Eur J Cardiothorac Surg 2015;47:e131-9. [Crossref] [PubMed]
- Sortini D, Feo C, Maravegias K, et al. Intrathoracoscopic localization techniques. Review of literature. Surg Endosc 2006;20:1341-7. [Crossref] [PubMed]
- Sekimura A, Funasaki A, Iwai S, et al. Thoracoscopic small pulmonary nodule detection using computed tomography-guided cutaneous marking and pleural marking. J Thorac Dis 2019;11:2745-53. [Crossref] [PubMed]
- Sakiyama S, Kondo K, Matsuoka H, et al. Fatal air embolism during computed tomography-guided pulmonary marking with a hook-type marker. J Thorac Cardiovasc Surg 2003;126:1207-9. [Crossref] [PubMed]
- Yi JH, Choi PJ, Bang JH, et al. Systemic air embolism after computed tomography-guided hook wire localization: two case reports and literature review. J Thorac Dis 2018;10:E59-64. [Crossref] [PubMed]
- Nishida T, Fujii Y, Akizuki K. Preoperative marking for peripheral pulmonary nodules in thoracoscopic surgery: a new method without piercing the pulmonary parenchyma. Eur J Cardiothorac Surg 2013;44:1131-3. [Crossref] [PubMed]
- Kamiyoshihara M, Ibe T, Kawatani N, et al. A convenient method for identifying a small pulmonary nodule using a dyed swab and geometric mapping. J Thorac Dis 2016;8:2556-61. [Crossref] [PubMed]
- Mun M, Matsuura Y, Nakao M, et al. Noninvasive computed tomography-guided marking technique for peripheral pulmonary nodules. J Thorac Dis 2016;8:S672-6. [Crossref] [PubMed]
- Okuda K, Yano M, Sasaki H, et al. A safe method for marking small pulmonary nodules with crystal violet. Surg Today 2015;45:871-5. [Crossref] [PubMed]
- Belavy D, Sunn N, Lau Q, et al. Absence of neurotoxicity with perineural injection of ultrasound gels: assessment using an animal model. BMC Anesthesiol 2013;13:18. [Crossref] [PubMed]