
A multidisciplinary approach for the diagnosis of benign asbestos pleural effusion: a single-center experience
Introduction
Benign asbestos pleural effusion (BAPE) is a type of asbestos related lung disease (ARLD). It is a rare non-malignant pleural disease first described in 1964 (1). It is a frequent manifestation of asbestos exposure in the pleura. In China, asbestos exposure was generally high, although it has decreased over the last few decades (2). We are now experiencing an epidemic of ARLD that is the legacy of occupational exposure of the last few decades. The reported prevalence of BAPE is not clear, however it has likely increased over time (3,4).
In 1982, Epler et al. (4). advocated diagnostic criteria for BAPE. Later, Hillerdal and Ozesmi (5) described some additions and modifications to these criteria. According to the criteria, an asbestos exposure history is critical for the diagnosis, however the interval between initial exposure and the disease varies greatly from between 5.5 up to 28 years (6,7). The clinical characteristics of BAPE are always based on case reports or case series and seem to be non-pathognomonic. Typically, patients with BAPE have no symptoms, while symptomatic patients may develop acute inflammatory pleuritis with chest pain, fever, and bloody pleural fluid (PF). Although an increasing number of cases have been reported recently, few prospective studies are available (6,8,9). Therefore, the diagnostic approach for BAPE is challenging and remains unclear to physicians. Delayed diagnosis or misdiagnosis may lead to significant morbidity and potential mortality (8,9).
To better characterize BAPE and outline its diagnostic processes, the comprehensive clinical data of 11 consecutive patients with BAPE was collected and analysed in the prospective study. Importantly, a multidisciplinary practical diagnostic approach for BAPE was introduced.
Methods
Patients
Four hundred and 82 consecutive patients who were treated at Guangzhou Institute of Respiratory Health, the First Affiliated Hospital of Guangzhou Medical University between January 2013 and January 2018 were scanned using high-resolution chest CT and had PF, but those without asbestos exposure were excluded from this study. Twenty-one suspected cases with BAPE were identified but only 11 had complete clinical information. Therefore, a total of 11 patients were included in the study. The inclusion criteria (4,5) for enrolment of the patients were as follows: (I) asbestos exposure; (II) exudative effusion; (III) PF confirmed by chest CT with or without other ARLD [pleural plaques, diffuse pleural thickening (DPT)]; and (IV) at least 2-year follow-up assessments. Other pleural diseases such as lung cancer, malignant pleural mesothelioma (MPM), and tuberculous pleuritis were excluded after pleural biopsy, pleuroscopy, cytological analysis and laboratory testing. The study was approved by the institutional Review Board of the First Affiliated Hospital of Guangzhou Medical University. Informed consent was obtained from all participants. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Exclusive diagnosis
BAPE is diagnosed by exclusion. Prior to further laboratory testing and imaging examination for BAPE, it is necessary to review the patient’s asbestos exposure, medical and surgical history to identify the potential causes. The common aetiology of PF including malignant pleural effusion (MPE), tubercular pleural effusion (TPE), parapneumonic pleural effusion (PPE) and pleural parasitic infestation (PPI) (10) were excluded by laboratory tests (11).
Pleural biopsy and thoracentesis procedure
Pleural samples were acquired by combined ultrasound-guided cutting needle biopsy and standard pleural biopsy (11). Thoracentesis was performed by trained chest physicians using the standard technique. The procedure was suspended if spontaneous cessation of fluid drainage occurred or if the patient feel discomfort with exacerbation of symptoms or vagal manifestations.
Laboratory testing
Biochemical analysis, bacterial, fungal and mycobacterial culture, gram staining, and cytological examinations were performed for all PF samples using standard methods.
Multidisciplinary team (MDT) members and approach for BAPE diagnosis
MDT members included physicians, thoracic radiologists, and pathologists. They discussed a suspected BAPE case from their specialty perspectives. At least 2-year follow-up was conducted if nonspecific treatment was taken.
Data analysis
The complete clinical information of 11 patients with BAPE were prospectively collected and analysed. This included symptoms, asbestos exposure history, laboratory findings, diagnostic process and follow-up. Continuous variables were presented as median and range, and qualitative variables as numbers and percentages.
Results
Characteristics of patients with BAPE
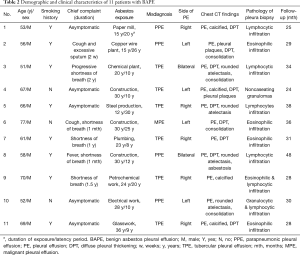
MDT members and their roles in the diagnosis of BAPE are listed in Table 1. In total, the complete clinical data sets of 11 patients with BAPE were collected and analysed. The clinical characteristics of the 11 patients with BAPE are summarized in Table 2. The patients were all men with a median age of 61.8 years (range, 51–77 years). All the patients had a history of asbestos exposure and the median exposure duration was 24 years (range, 12–36 years). Among the patients, 8 (72.7%) had a smoking history. Unilateral effusions were found in 9 (81.8%) and bilateral effusions were detected in the remaining of the patients (18.2%). Six patients had respiratory symptoms including shortness of breath (n=4), cough (n=2), fever (n=1) and excessive sputum (n=1). The duration of these symptoms ranged from 2 weeks to more than 2 years. However, the other 5 patients (5/11, 45.5%) were asymptomatic. Pulmonary physical examination revealed remarkably decreased breath sounds with dullness to percussion on the lateral or bilateral chests, without significantly positive signs. Six patients with BAPE were initially misdiagnosed as TPE. The diagnoses of 4 patients was similar to PPE and another patient was considered as having MPE.

Full table
Full table
Laboratory tests for peripheral blood cell (PBC) and serological examination
All patients had PBC analysis and serological examinations. Leucocytosis of peripheral blood (>10×109/L) was observed in 3 cases (cases 2, 7 and 11), and eosinophilia (>0.5×109/L) was not seen in these patients. No specific findings were observed in the serological examination, including liver function, thyroid function, interferon-γ release assays (IGRAs) and brain natriuretic peptide (BNP). No detected indication of connective tissue disease, such as antinuclear antibody, rheumatoid factor antibody, proteinase 3, myeloperoxidase and anticyclic citrullinated peptide antibody, were found. Carcinoembryonic antigen (CEA) was negative. Sputum smears and cultures for fungi, acid-fast bacilli and other bacteria were also negative. In addition, the test for parasite-specific IgG antibody was negative, and parasite eggs in the stool were not found in any of the stool samples. The results of the serological examination are summarized in Table 3.
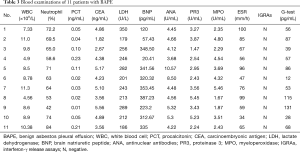
Full table
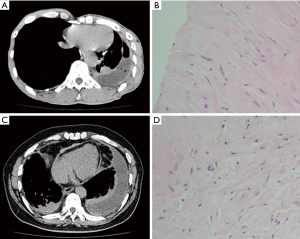
Chest CT scans were performed on all patients. PF was found in all patients, in addition, pleural plaques, DPT, asbestosis, and round atelectasis were found in cases 5, 2, 1, and 1, respectively (Table 2). CT images of typical cases (cases 1 and 3) of BAPE prior to thoracentesis are shown in Figure 1.
Invasive work-up
After routine tests for serological examination and chest CT scan, a thoracentesis and pleura biopsy were performed. PFs were collected for further analysis in all patients. Five patients had intrapulmonary involvement and intrapulmonary lesions presented as consolidation. Bronchoscopy and transbronchial lung biopsy (TBLB) were performed in these patients, and there was no evidence of tuberculosis or malignancy.
Pleural parameters in PF
Bloody effusion was found in one patient and eosinophilic pleural effusion (EPE) was confirmed in this patient; there were 11% of eosinophils in the PF (case 1). The median concentrations of lactate dehydrogenase (LDH), adenosine deaminase (ADA), proteins and CEA in the PF were 221.4 U/L (range, 189.8–11,325 U/L), 21 U/L (rang, 14–247 U/L), 48.3 g/dL (range, 35.2–53.2 g/dL) and 3.46 mg/mL (range, 0.84–4.54 mg/mL), respectively (Table 4). PF TB-DNA, acid-fast bacilli smears and pleural effusion culture for fungi or bacteria were negative. The pleural biopsy specimens indicated fibrosis of the pleura, mesothelial cells, and lymphocytes (Figure 1). Asbestos bodies (ABs) and asbestos fibers were not found, and there was no evidence of tuberculosis or malignancy.
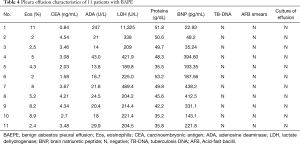
Full table
Moreover, positron emission tomography/computed tomography (PET/CT) was used to exclude the diagnosis of MPE (case 6).
Non-specific but symptomatic treatment after the initial diagnosis of BAPE
The MDT discussed with the suspected BAPE, non-specific but symptomatic treatment was performed with strict follow-up after the initial diagnosis of BAPE. Physical examination with chest radiography, ultrasound and/or CT were conducted during the follow-up. The follow-up was ≥24 months (mean 31.9 months, range, 24–48 months). All patients were in stable condition during the follow-up and did not receive any additional therapy. One patient showed regression of the PF. Figure 1 showed the follow-up chest CT of one of the patients (case 3).
MDT approach to diagnosing BAPE
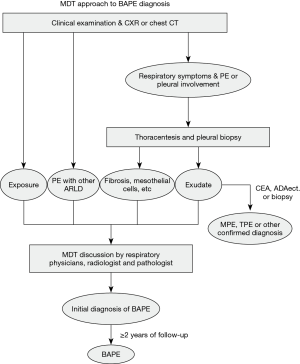
Multidisciplinary discussion involves several disciplines including respiratory specialties, pathology and radiology. Each of MDT members expressed his/her own opinions, and disputes were discussed until a consensus was reached. Their collaborative work culminated into the final diagnosis of a patient. A practical diagnostic approach for BAPE was developed and is shown in Figure 2.
Discussion
Data regarding BAPE comes primarily from a few case reports and a small series of patients (6,8,9). Early in 1996, Ferrer et al. (9). reported on 15 patients with BAPE in Spain. In 2015, Fujimoto et al. (6). retrospectively analysed the clinical features of BAPE in patients in Japan. Kato et al. (12). evaluated the pleural irregularities and pleural involvement of BAPE compared to MPM. However, no satisfactory diagnostic approach has been proposed and the diagnosis of BAPE remains a problem in contemporary clinical practice. In the current study, an MDT approach for the diagnosis of BAPE was introduced after summarizing the clinical features and laboratory data of 11 cases of BAPE patients.
We attempted to describe the clinical features of BAPE in 11 cases that were prospectively collected. An asbestos exposure history is one of the important diagnostic criteria for BAPE (4,5). However, the relationship with the type of occupation and the median latency period between asbestos exposure and BAPE development varied greatly. In a clinical investigation, Fujimoto et al. (6). found that in cases of shipbuilding (25/108), construction (20/108) and chemical facility (10/108) workers BAPE accounted for more than half of the cases. In previous studies (6,7,13), the interval between exposure and presentation of BAPE varied from 5 years to more than 30 years. In the present study, all patients had a remarkable occupation exposure history. Construction was the most common occupational exposure (3/11), the median duration was 24 years (range, 12–36 years) and the interval between exposure and the presentation of BAPE was 12 years (range, 8–30 years). The insidious onset of dyspnoea is the most common respiratory symptom associated with BAPE, typically beginning with exertional dyspnoea (14). Asymptomatic patients were more frequently (5/11, 45.5%) observed in our study than in previous reports (35/110, 31.8%) (6). Acute symptoms (such as fever) were also found in our study (case 8), and leucocytosis (>10×109/L) was also observed in 3 cases (cases 2, 7 and 11). Without careful assessment, these patients were easy to be diagnosed as PPE. Most patients had bilateral PF; however, some had only unilateral effusion (5). However, in our study, unilateral effusions were seen in 9 patients (81.8%) and bilateral effusion was seen in the remaining patients (18.2%). Because of the lack of awareness, non-specific clinical characteristics and laboratory tests, most patients were initially misdiagnosed.
Current investigations on pleural effusions emphasize the use of a diagnostic algorithm or recommend the use of a stepwise approach (15,16). Thoracocentesis was performed to ascertain the nature of the PF and differentiate it from other conditions. Pleural effusions in the 11 patients were exudative according to Light’s criteria. Pleural effusions secondary to BAPE always can be presented as a hemorrhagic, lymphocytic exudate, with occasional eosinophilic fluid (5). The pleural effusion parameters were seen to be non-specific and extremely variable. The concentration of CEA in PF is a representative tumor marker and it plays a role in MPE differentiation. It is often positive in highly suspicious cases of MPE, although a normal level of CEA can also be found in malignant conditions (17). In this study, CEA in the serum and PF were observed at normal levels (<5 mg/mL). Other PF parameters, such as ADA and LDH, had rarely been evaluated in previous studies (5,6). In our study, the median level of fluid LDH ranged from 189.8 to 11,325 U/L. Therefore, prospective studies with a larger number of patients are required to investigate the actual diagnostic value of specific PF parameters.
When there is clinical suspicion of BAPE, obtaining a pleura biopsy specimen is mandatory. Either thoracoscopy or closed percutaneous pleural biopsy are acceptable and safe methods (14). However, in some cases, for the symphysis of the parietal and visceral pleura, attempting a thoracoscopy may fail. In this study, pleural samples were collected using a combined ultrasound-guided cutting needle biopsy and a standard pleural biopsy, without thoracoscopic assessment. Sufficient pleura biopsies were obtained. The sensitivity and accuracy reached up to 88.6% and 93.8%, respectively (12), which were close to thoracoscopy examinations (11). Contrary to what might be expected, ABs or asbestos fibres are not usually found in the pleura, whilst they can be deposited in the lungs and sputum. Fibrosis of the pleura was considered to be the indirect pleural effects from the pulmonary asbestos fibres in the case reports (18).
The diagnosis of BAPE is based on an asbestos exposure history, exudative effusion and an exclusion of other causes of the effusion. Because of its’ obscure exposure history, nonspecific clinical characteristics and PF, the definite diagnosis of BAFE has significant challenges. The diagnostic approach is rarely discussed; therefore a multidisciplinary assessment of its diagnosis is a valid approach. Here, we highlight the value of MDT discussion of the diagnosis of BAPE.
In our present study, the clinicians collected clinical data when BAPE was suspected, an MDT approach was performed in the identified patients. The radiologists focus on other characteristics of benign asbestos pleural disease (pleural plaques, DPT and/or rounded atelectasis) excepting PF. When the physicians suspected BAPE. The presence of other radiologic characteristics of benign asbestos pleural disease assists in distinguishing BAPE from other idiopathic effusions. Some patients in our series combined with pleural plaques, and/or DPT, rounded atelectasis. When excessive pleural fibrosis was discovered in pleural specimens by a pathologist, a wide of other conditions (e.g., after tuberculous pleuritis, empyema, hemothorax, or irradiation) required exclusion. This was accomplished by discussing the clinical characteristics with all clinicians. After careful exclusion of alternative aetiologies, an optimized diagnosis was made to avoid unnecessary testing (e.g., internal thoracoscopy) and optimise patient management. The MDT approach for BAPE seemed to be practical in our study and the 11 patients were diagnosed after follow-up for ≥2 years.
This study had limitations. Because this was a single-center experience with a small number of patients, the characteristics BAPE were not fully addressed. A large, multicenter, prospective study is required to validate our findings.
In conclusion, in patients with unexplained PF, a high level of suspicion for BAPE should be considered, especially when combined with pleural plaques, DPT, and rounded atelectasis. The MDT-based diagnostic approach may allow clinicians to avoid the limitations of individual diagnosis and manage patients with a high degree of confidence.
Acknowledgments
Editorial assistance with the manuscript was provided by Content Ed Net, Shanghai Co. Ltd.
Funding: None.
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1119
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1119). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (No. 2013012). All patients provide written informed consent at recruitment. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Eisenstadt HB. Asbestos pleurisy. Dis Chest 1964;46:78-81. [Crossref] [PubMed]
- Ramazzini C. Asbestos is still with us: Repeat call for a universal ban. Am J Ind Med 2011;54:168-73. [Crossref] [PubMed]
- Prazakova S, Thomas PS, Sandrini A, et al. Asbestos and the lung in the 21st century: an update. Clin Respir J 2014;8:1-10. [Crossref] [PubMed]
- Epler GR, McLoud TC, Gaensler EA. Prevalence and incidence of benign asbestos pleural effusion in a working population. JAMA 1982;247:617-22. [Crossref] [PubMed]
- Hillerdal G, Ozesmi M. Benign asbestos pleural effusion: 73 exudates in 60 patients. Eur J Respir Dis 1987;71:113-21. [PubMed]
- Fujimoto N, Gemba K, Aoe K, et al. Clinical Investigation of Benign Asbestos Pleural Effusion. Pulm Med 2015;2015:416179.
- Ernst P, Zejda J. Pleural and airway diseases associated with mineral fibers. In: Lidderll D, Miller K. editors. Mineral Fibers and Health, CRC, Boca Raton, FL, USA, 1991:121-34.
- Hiyama J, Marukawa M, Shiota Y, et al. Benign asbestos pleural effusion associated with pulmonary aspergilloma. Intern Med 1998;37:965-8. [Crossref] [PubMed]
- Ferrer J, Balcells E, Orriols R, et al. Derrame pleural benigno por asbesto. Descripción de la primera serie en España. Med Clin (Barc) 1996;107:535-8. [PubMed]
- Wang J, Luo W, Shen P, et al. Retrospective study of pleural parasitic infestations: a practical diagnostic approach. BMC Infect Dis 2019;19:576. [Crossref] [PubMed]
- Wang J, Zhou X, Xie X, et al. Combined ultrasound-guided cutting-needle biopsy and standard pleural biopsy for diagnosis of malignant pleural effusions. BMC Pulm Med 2016;16:155. [Crossref] [PubMed]
- Kato K, Gemba K, Fujimoto N, et al. Pleural irregularities and mediastinal pleural involvement in early stages of malignant pleural mesothelioma and benign asbestos pleural effusion. Eur J Radiol 2016;85:1594-600. [Crossref] [PubMed]
- Robinson BW, Musk AW. Benign asbestos pleural effusion: diagnosis and course. Thorax 1981;36:896-900. [Crossref] [PubMed]
- American Thoracic Society. Diagnosis and initial management of nonmalignant diseases related to asbestos. Am J Respir Crit Care Med 2004;170:691-715. [Crossref] [PubMed]
- Hooper C, Lee YC, Maskell N, et al. Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii4-ii17. [Crossref] [PubMed]
- McGrath EE, Blades Z, Needham J, et al. A systematic approach to the investigation and diagnosis of a unilateral pleural effusion. Int J Clin Pract 2009;63:1653-9. [Crossref] [PubMed]
- Yang Y, Liu YL, Shi HZ. Diagnostic Accuracy of Combinations of Tumor Markers for Malignant Pleural Effusion: An Updated Meta-Analysis. Respiration 2017;94:62-9. [Crossref] [PubMed]
- Mutsaers SE, Prele CM, Brody AR, et al. Pathogenesis of pleural fibrosis. Respirology 2004;9:428-40. [Crossref] [PubMed]


