
Quantifying the learning curve of emergent total arch replacement in acute type A aortic dissection
Introduction
Acute type A aortic dissection is a lethal condition with considerable morbidity and mortality. Forty percent of patients die immediately and do not reach medical providers. Without treatment, the mortality increases as much as 1–2% per hour during the first 24 to 48 hours (1-3). Over 70% of acute type A dissections extend distally into the aortic arch and descending thoracic aorta (4). Emergent total arch replacement with stented elephant trunk is often necessary in the acute setting to restore cerebral, visceral, and/or peripheral perfusion. However, operations involving the aortic arch are technically demanding, especially in acute aortic dissection, further complicated by a high incidence of concomitant aortic root dissection requiring intervention (5).
Results following acute type A aortic dissection repair are known to be case-load and surgeon dependent (6). However, the learning curve has never been systematically investigated. It is unclear whether new faculty surgeons are able to achieve high performance and acceptable outcomes in their start-up period.
The utility of the sequential probability cumulative sum (CUSUM) technique to analyze surgical performance has been reported (7-11). It allows for construction of a learning curve, demonstrating surgeon-specific incidence of perioperative complications and mortality. CUSUM has been used to analyze the learning curve in aortic dissection in previous publication (12). However, the sample size was small with 30 patients over a seven-year period, which may be insufficient to draw reliable conclusions. In most institutions, emergent operations for acute aortic dissection are commonly performed by the on-call cardiac surgeons, some of whom may be relatively newly appointed faculty. We sought to determine whether their performance and outcomes were consistent and acceptable in the start-up period through a systematic investigation.
As a tertiary medical center, our institution has a high-volume aortic surgery program. During last few years, over 100 patients with acute type A aortic dissection with arch involvement were treated surgically each year. From January 2013 to December 2016, 300 cases of emergency total arch replacement were performed in our institution by three surgeons, including a surgeon who had extensive experience in aortic dissection before the time frame of this study. The other two surgeons entered this program since 2013. This unique situation presented an opportunity for our program to an optimal setting to conduct a CUSUM analysis for acute dissection repair.
We present the following article in accordance with the “Strengthening The Reporting of Observational Studies in Epidemiology (STROBE) Statement” reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-912).
Methods
Study population and preoperative evaluation
From January 2013 to December 2016, consecutive 382 patients with acute type A aortic dissection underwent emergent aortic repair at our institution. Patients with aortic arch involvement who underwent total arch replacement were enrolled into this study. Patients with acute myocardial infarction, recent stroke related to acute dissection, cardiac tamponade, visceral or peripheral malperfusion, hypoxia requiring mechanical ventilation, and history of previous open-heart surgery were excluded. A total of 300 patients formed the study group. We divided the entire cohort into three groups by the operative surgeon. Baseline characteristics, procedural and postoperative outcomes were compared among these groups. In order to study the evolution process of a single surgeon’s performance longitudinally, we divided the patients operated by Dr. A into four groups by case number.
Surgical technique
All cases were performed under deep hypothermic circulatory arrest (DHCA) with anterograde selective cerebral perfusion. Bilateral percutaneous cerebral oxygenation saturation monitoring was routinely used. During the operation, patient was placed in the supine position with the right arm extended. Right (or left) common femoral artery and right axillary artery were cannulated. Middle sternotomy was performed. Right atrium appendage was cannulated to complete the establishment of cardiopulmonary bypass (CPB). Retrograde cannula was inserted into coronary sinus through the right atrium. After the ascending aorta was cross-clamped and opened, Del Nido cardioplegia solution was given directly through coronary ostia followed by intermittent retrograde perfusion every 60 to 90 minutes. In the case of dissection involving coronary ostium, retrograde cardioplegia perfusion was used alone.
During cooling, the aortic root was dissected and inspected. The root was addressed using different techniques at the discretion of the operating surgeon. The techniques used included patch reinforcement, valve-sparing aortic root replacement (VSRR) with reimplantation technique, Bentall procedure and sinus of Valsalva repair.
DHCA was instituted after the nasopharyngeal temperature reached 20 to 22 °C. Unilateral cerebral perfusion was administrated at 10 mL/kg/min through right axillary artery. The entire aortic arch was resected. The innominate artery, the left common carotid artery and the left subclavian artery were amputated in proximal segments. It was at surgeon’s discretion whether or not a stent elephant trunk (SET) repair was needed. A stent graft (MicroPort Medical Co Ltd, Shanghai, China) was used for SET repair. A 4-branched graft (Gelweave ValsalvaTM TERUMO, Tokyo, Japan) was used to replace the total arch. The distal arch anastomosis was performed first. The graft was cross clamped at the ascending aorta level and the femoral artery perfusion restarted. Then the left common carotid artery anastomosis was completed and rewarming initiated. At this time, the distal perfusion was translocated from the femoral artery to the 4th branch of the graft. During the circulatory arrest, the cerebral perfusion would be revised if the cerebral oxygenation saturation decreased for more than 20 in absolute value or 40% from the baseline.
During rewarming, the left subclavian artery was anastomosed. The root repair as well as the proximal aortic anastomosis were completed, and aortic cross-clamp removed. Then, the innominate artery anastomosis was completed. The patient was weaned off the CPB and the hemostasis was achieved.
In case of difficulty in coming off bypass, a transesophageal echocardiogram (TEE) was performed to evaluate the ventricular and valvular function. It was at surgeon’s discretion whether or not to perform CABG if the ventricular dysfunction was verified by TEE and was deemed to be related with compromised coronary blood flow. The CABG was performed under on-pump beating heart technique with saphenous vein.
After coming off bypass, if the systolic pressure of the lower limb was significantly less than that in the ascending aorta (difference ≥30 mmHg), an ascending aorta-iliac artery bypass was performed with a 10 mm graft (Gelweave ValsalvaTM TERUMO, Tokyo, Japan) at surgeon’s discretion.
Data collection
Data on all preoperative, intraoperative, and postoperative variables were obtained from our cardiovascular surgical database or manually collected from the electronic medical record at Zhongshan Hospital, Fudan University, Shanghai, China.
Study endpoints
The primary endpoint was operative mortality, which was defined as death in hospital or within 30 days after surgery. The secondary endpoints included stroke as well circulatory arrest time and cross-clamp time. The circulatory arrest period consisted of femoral artery perfusion cessation to reinitiation. Prolonged mechanical ventilation was defined as requiring mechanical ventilation for more than 72 hours after surgery.
CUSUM analysis
Cumulative time and failure charts and their usage in depicting a surgical learning curve have been descripted in previous publications (7,11,13). The statistical principles were adapted from the comprehensive introduction in previous publication (8).
To compute the learning curves in regard to the time of circulatory arrest and cross-clamp, we plotted the CUSUM of difference of each value from the mean circulatory arrest and cross-clamp time. Since we had a surgeon with much larger case volume than the other two, his performance might influence the shape of others’ learning curve significantly in case of using the mean value of the entire cohort. So, we decided to depict two different sets of curves using the mean time derived either from the entire group or from every single surgeon, respectively.
In cumulative failure charts, the CUSUM was defined as Sn = where Xi = 1 for a “failure” (operative mortality) and as Xi = 0 for a “success” (no operative death). The target value p0 was set to 0.08, indicative of an “acceptable failure rate” of 8% according to the mortality reported in previous publications (14,15). The control boundaries were plotted according to the formulas described in previous publications (7,11). A CUSUM curve that reaches an upper boundary indicates an unacceptable failure rate as p1=0.12. We set 80% of the boundary line as an alert line while 95% as an alarm line which indicate the need for thorough investigation. Crossing below the lower 80% alert line indicates results better than target values.
Ethics
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The data were approved for use in research by the Zhongshan Hospital Institutional Review Board (IRB), with patient consent waived.
Statistical analysis
Normally distributed continuous variables are presented as mean ± standard deviation and categorical variables, as number and percentage. For comparison of continuous variables independent sample analysis of variation (ANOVA) was applied. For comparison of categorical variables, the Chi-square test or Fisher exact test (when appropriate) was used. A P value less than 0.05 was considered significant. The statistical package used was SPSS software (Version 22; IBM Corp, New York, NY, USA). The logarithmic regression was performed using Prism (Version 8.2.1; GraphPad Software, San Diego, CA, USA). All the charts were plotted using Prism as well.
Results
Demographics and baseline characteristics
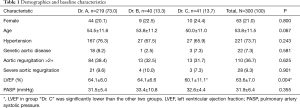
From January 2013 to December 2016, 382 patients with acute type A aortic dissection underwent emergency aortic repair in our institution. A total of consecutive 300 patients (age 53.8±11.5 years, female 63, 21.0%) underwent emergency total arch replacement were enrolled in this study. They were grouped by operative surgeons (Drs. A, B and C). Two hundred and twenty-one patients (73.7%) had systemic hypertension. Twenty-two patients (7.3%) were deemed to have Marfan’s syndrome or another genetic aortic disease according to physical examination. One hundred and ten patients (36.7%) had aortic regurgitation more than mild degree, 28 patients (9.3%) had severe aortic regurgitation secondary to commissure detachment. The demographics and baseline characteristics were balanced among groups except left ventricular ejection fraction (LVEF), which was significantly lower in group “Dr. C” than the other groups, although LVEF was in normal range in all groups. The details are demonstrated in Table 1.
Full table
There were 219 patients operated by Dr. A. They were divided into four evenly distributed groups (T1 to T4). T1 (n=54) spanned from January 2013 to December 2013, T2 (n=55) spanned from December 2013 to October 2014, T3 (n=55) spanned from November 2014 to September 2015, T4 (n=55) spanned from October 2015 to December 2016.
Procedural outcomes
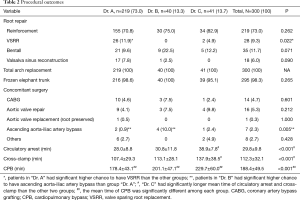
All patients underwent total arch replacement. Two hundred and nineteen patients (73.0%) had aortic root reinforcement with supracoronary ascending aorta replacement. Two hundred and ninety-five patients (98.3%) underwent frozen elephant trunk repair. Patients in “Dr. A” group had a higher chance to undergo VSRR than patients in the other two groups.
Fourteen patients (4.7%) underwent CABG after having difficulty in coming off bypass, which was deemed to be related to compromised coronary blood flow resulting from unsatisfactory reconstruction of dissected coronary ostia. Seven patients (2.3%) underwent ascending aorta-iliac artery bypass because of compromised blood flow to the lower limbs, which might be a result of true lumen occlusion or stenosis. Seventeen patients (5.6%) underwent aortic valve repair or replacement without root replacement. Other concomitant surgeries included mitral or tricuspid valve repair, atrial septal defect (ASD) or patent foramen ovale (PFO) repair, aortic root-right atrium shunt and right axillary embolism retrieval. When compared to “Dr. A”, patients in group “Dr. B” had a higher chance to have ascending aorta-iliac artery bypass.
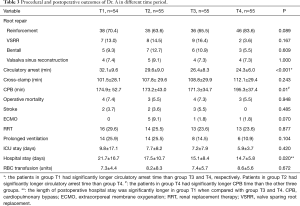
The mean time of circulatory arrest, cross-clamp and CPB were 29.8±9.8, 112.3±32.1 and 188.4±49.5 min, respectively. The “Dr. C” group had significantly longer mean time of circulatory arrest and cross-clamp than the other two groups. The mean time of CPB were significantly different among each group, as “Dr. A” had the shortest and “Dr. C” had the longest time. The procedural outcomes were studied longitudinally in patients operated by Dr. A, separately. Significant trend of shorter length of circulatory arrest time was observed over the time period from T1 to T4.
The details of procedural outcomes are shown in Tables 2 and 3.
Full table
Full table
Postoperative outcomes
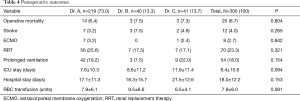
The operative mortality and stroke rate were 6.7% (20 patients) and 4.0% (12 patients), respectively. Eight patients (2.7%) underwent venous-venous extracorporeal membrane oxygenation (ECMO) insertion due to refractory severe hypoxia. The mean length of postoperative ICU and hospital stay were 8.4±10.6 and 18.0±12.2 days, respectively. There was no significant difference in operative mortality or other complications among each group. The postoperative outcomes were studied longitudinally in patients operated by Dr. A, separately. Significant trend of shorter length of postoperative hospital stay was observed over the time period from T1 to T4.
The details of postoperative outcomes are shown in Tables 3 and 4.
Full table
Time of critical operative steps
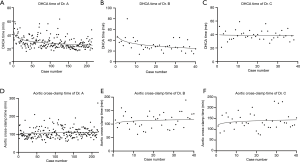
The details of the mean time of critical operative steps in each group are shown in Table 2. The learning curves regarding to circulatory arrest time were calculated using logarithmic regression, which are demonstrated in Figure 1. The trend graphs of circulatory arrest time of Dr. A and B confirmed the presumed shape with a relatively steep slope at the beginning, which indicated the progressive process within a short period of learning, while the graph of Dr. C demonstrated a constant stable process (Figure 1A,B,C). The subgroup analysis of Dr. A showed a significant trend of shorter circulatory arrest time over time (Table 3). The trend graphs of cross-clamp time of each surgeon were made as well, however, the values can’t fit into the regression model quite well due to high unpredictable of this variable (Figure 1D,E,F).
CUSUM operative time depiction
CUSUM learning curves regarding the time of critical operative steps were plotted. CUSUM of the difference in circulatory arrest and cross-clamp time from the mean values were plotted on the vertical axis, respectively. Given Dr. A had a much larger case volume than the other two surgeons, shape of the learning curves of the other two surgeons might be distorted significantly if the mean values were derived from the entire cohort. For this reason, we plotted the curves based on the mean value derived from the entire group and from every single surgeon, respectively, to demonstrate and eliminate the influence by high-volume Dr. A (Figure 2).
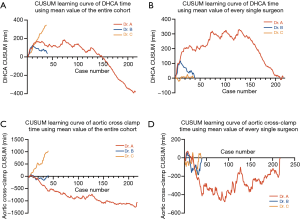
In case of using the mean value derived from the entire cohort, both the CUSUM of circulatory arrest and cross-clamp time of Dr. C kept increasing, because the time in Dr. C’s cases were almost always longer than the mean value of the entire cohort (Figure 2A,C). While in case of using the mean value derived from every single surgeon, the progressive processes were demonstrated in Dr. A and B, while the performance of Dr. C appeared to be constant stable from the very beginning (Figure 2B,D). The reflection points in circulatory arrest time learning curves of Dr. A and B were 75 cases and 10 cases, respectively (Figure 2B).
CUSUM failure analysis of operative mortality
The cumulative failure charts of each surgeon were demonstrated in Figure 3. The “failure” was defined as death in hospital or within 30 days after surgery. No surgeon passed the upper “alert” or “alarm” line, which indicated the mortality for each surgeon was acceptable during the whole study time frame. Dr. A had the largest case volume in this study, as he had been performing complex aortic surgery on a regular basis for more than 15 years, which result not only shorter operative time but also relatively lower mortality. He broke and kept bellowing the lower 80% alert line after the 69 cases, which indicated superior results of his operations.

Discussion
Acute type A aortic dissection is a life-threatening condition, which often requires emergency surgery. The most widely accepted technique consists of resection of the primary entry tear, replacement of the ascending aorta and “hemi-arch” with open distal anastomosis, combined with aortic valve resuspension or Bentall procedure to address the dissected aortic root (16). Historically, this technique was believed to give patients the best chance to survive, even in the hands of non-specialized cardiac surgeon. The potential aortic complication or reintervention rates were assumed to be not significantly different between total arch replacement and “hemi-arch” (17,18).
However, the strategy of arch management in this setting remains controversial. Some studies demonstrate similar postoperative mortality and morbidity between total arch replacement and conservative arch management (18,19). A recent meta-analysis showed relative lower re-operation rate in total arch replacement, albeit not significant (20). The mean age of patients in our study was 53.8±11.5 years. Consistent with other studies from China, our patients were relatively younger than those reported from western countries (19,21-23). In decision making, age of the patient was an important concern. Younger patients are relatively healthier and seem to derive more long-term benefit with aortic arch replacement.
Unquestionably, total arch replacement combined with root repair is technically demanding. Surgeon experience may be an important factor for early outcomes. However, the time for personnel and other resources mobilization is limited in an emergency setting. Our clinical question asked whether an adult cardiac surgeon could achieve consistent performance and acceptable results in their start-up period of operating on acute dissection patients independently. As a tertiary adult cardiac surgery center, we had a surgeon with experience of emergency dissection repair for more than a decade, and two other surgeons who also had plenty of experience in routine cardiac surgery but only assisted in emergency dissection repair before time of this study. We believed these features make our program a good sample to conduct a research to answer the question we concerned about.
All patients in this study underwent total arch replacement with almost uniform techniques. However, the mean circulatory arrest time of Dr. C was significantly longer than those of Dr. A and B. The difference related to time devoted to the distal arch anastomosis. Dr. C modified this anastomosis technique by adding another layer of pericardial patch to help improving hemostasis. In scatter plot charts of circulatory arrest with logarithmic regression (Figure 1A,B), Dr. A and B confirmed the presumed shape with a relatively steep slope at the beginning, which indicated the progressive process within a short period of learning, while the graph of Dr. C demonstrated a constant stable process (Figure 1C). In subgroup analysis, a significant trend of shorter circulatory arrest time over time was observed in patients operated by Dr. A. It is easily to understand that as surgeon’s experience accumulated. The different trend in graphs of circulatory arrest time demonstrated that different surgeons may present their unique learning process as they may modified their techniques in a personal way (Figure 1A,B,C). Dr. C also had the longest mean cross-clamp time because of the same reason. We could not get an ideal regression equation for the cross-clamp time (Figure 1D,E,F), which meant the cross-clamp time was more unpredictable. This could be explained by the variation in complexity of root repair technique.
We did not depict the scatter plot graph for CPB time, because it was even more unpredictable than the cross-clamp time. In subgroup analysis of Dr. A, the patients in group T4 had significantly longer CPB time than the other three groups, however, factors including the time of rewarming, completing concomitant procedures, and achieving hemostasis had influences on the CPB time. As a result, the CPB time was not appropriate to represent the human factor from the surgeons.
Majority cases in each group underwent aortic root reinforcement to resuspend the torn aortic valve commissures and eliminate the dissected root. While 26 patients (11.9%) in Dr. A’s group had their native aortic valve preserved through reimplantation technique, the rate was significantly higher than the other two groups. Obviously, performing a VSRR during a total arch replacement for acute aortic dissection is technically demanding and risk accompanying. Given Dr. A had rich experience in this field, he could be confident to use this complex technique in such a challenging situation. All patients underwent VSRR had aortic regurgitation no more than mild degree before they left the operating room. We believed VSSR is a feasible and reliable technique for selected cases in experienced hands, however, for surgeons in their start-up period, Bentall should be the first choice for extended root dissection and root aneurysm.
The overall operative mortality in this study was 6.7%. Dr. A had the lowest mortality of 6.4%, albeit not significantly. The operative mortality for emergency acute type A aortic dissection repair varied among literatures from 3.9% to 24.1% in different eras (15,19,22). Dr. B and Dr. C finished their training in adult cardiac surgery and became staff surgeons at least one year before getting involved into the acute dissection team. They were experienced in general adult cardiac surgery with personal case volume of 150–200 each year for other kinds of adult cardiac surgery rather than acute dissection before this study. Our results demonstrated that even with the presence of new hands, who had been trained in adult cardiac surgery, the operative mortality was still acceptable although the time of operation would be longer.
In subgroup analysis of Dr. A, the postoperative outcomes including morbidity and mortality were almost constant over the time periods, which indicated the stable performance of Dr. A as an experienced surgeon. We did observe a significant trend of shorter length of postoperative hospital stay over time, which could be attributed to the systemic improvement including postoperative management.
We depicted the CUSUM curves for differences of circulatory arrest and cross-clamp time from their mean values. Given Dr. A had the largest case volume which accounted for 73% of the entire cohort, his performance would have a great impact on the shape of the curves of the others. In case of using mean value derived from the entire cohort, the curves of Dr. B and C kept going upward, which failed to demonstrate the actual personal progressive process (Figure 2A,C). So, we decided to take the mean values derived from the entire cohort as well as from every single surgeon to get two different sets of graphs.
In case of using mean time of every single surgeon, it was reasonable that, the curves acclaimed in the first segment and descended close to zero, then oscillated around zero (Figure 2B,D). These curves could help to find out the reflection point of every surgeon, to determine the number of cases needed for a surgeon to achieve stable performance. According to CUSUM circulatory arrest time learning curve chart using the mean time of every single surgeon (Figure 2B), all surgeons appeared to have different patterns in their learning curves regarding to circulatory arrest time. The reflection points in circulatory arrest time learning curves of Dr. A and B were 75 cases and 10 cases, respectively. It did not mean that Dr. A need more cases to get a stable performance. The reasonable interpretation is that, the actual progressive process is staged. The experienced surgeon could get an even better result after a further successful modification of his technique. While for Dr. C, he had a relative constant circulatory arrest time from the very beginning, no reflection point could be calculated. The CUSUM cross-clamp time learning curve using mean time of every single surgeon (Figure 2D) presented in an oscillating fashion with no reflection points defined, which indicated that the cross-clamp was more unpredictable due to different combined surgery especially the way the root was addressed.
In CUSUM failure analysis, we defined the “failure” as operative mortality. Total arch replacement for patients with acute type A aortic dissection is a high-risk operation with high incidence of postoperative complications including stroke, acute renal failure and bleeding, etc. We did not define the “failure” to include these complications because they were highly associated with the involvement of aortic branches and the presence of end-organ malperfusion, and they were unable to represent the human factor from the surgeon. According to the CUSUM failure analysis chart, the curves of Dr. B and Dr. C stayed underneath the upper 80% alert line in their start-up period, which indicated their results were acceptable even with relative low volume (Figure 3B,C). While surgeon with large volume experience broke and kept underneath the lower 80% alert line after his 69 cases (it was not the 69th case in his career), which indicated the superior outcome and excellent performance.
The study has several limitations. Although the technique for total arch replacement in this study was almost uniform and the baseline characteristics among groups were substantially balanced, the heterogeneity of the patients cannot be eliminated. Especially the pathology of the root and the technique used to address it varied case by case. As a result, it was impossible to maintain “the degree of difficulty” in a constant level, which would influence the evaluation of the surgeon’s performance. Another limitation, Dr. A had a long history of performing aortic surgery, so the first case performed by him in this cohort was not truly the first one in his career, as he also operated in outside hospitals, those patients were not enrolled in this study. As a result, the learning curves of Dr. A cannot present his real progressive process, they could be used only as references to evaluate the performance of the other two surgeons.
Conclusions
The results of our study demonstrate that different surgeons have unique patterns of progression and learning. Although new surgeons have longer operative time, those with comprehensive training in adult cardiac surgery, can achieve consistent results and acceptable outcomes in their start-up period operating on acute type A aortic dissection with total arch replacement. Experienced surgeons are more likely to obtain better outcomes through further technique modifications.
Acknowledgments
Funding: This work was supported by National Natural Science Foundation of China Youth Science Foundation Project (81801743).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-912
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-912
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-912). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The data were approved for use in research by the Zhongshan Hospital Institutional Review Board (IRB), with patient consent waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Coady MA, Rizzo JA, Goldstein LJ, et al. Natural history, pathogenesis, and etiology of thoracic aortic aneurysms and dissections. Cardiol Clin 1999;17:615-35. [Crossref] [PubMed]
- Anagnostopoulos CE, Prabhakar MJ, Kittle CF. Aortic dissections and dissecting aneurysms. Am J Cardiol 1972;30:263-73. [Crossref] [PubMed]
- Masuda Y, Yamada Z, Morooka N, et al. Prognosis of patients with medically treated aortic dissections. Circulation 1991;84:III7-13. [PubMed]
- Shah A, Coulon P, de Chaumaray T, et al. Novel technique: staged hybrid surgical and endovascular treatment of acute Type A aortic dissections with aortic arch involvement. J Cardiovasc Surg (Torino) 2006;47:497-502. [PubMed]
- Urbanski PP, Lenos A, Irimie V, et al. Acute aortic dissection involving the root: operative and long-term outcome after curative proximal repair. Interact Cardiovasc Thorac Surg 2016;22:620-6. [Crossref] [PubMed]
- Bashir M, Harky A, Fok M, et al. Acute type A aortic dissection in the United Kingdom: Surgeon volume-outcome relation. J Thorac Cardiovasc Surg 2017;154:398-406.e1. [Crossref] [PubMed]
- Holzhey DM, Jacobs S, Walther T, et al. Cumulative sum failure analysis for eight surgeons performing minimally invasive direct coronary artery bypass. J Thorac Cardiovasc Surg 2007;134:663-9. [Crossref] [PubMed]
- Rogers CA, Reeves BC, Caputo M, et al. Control chart methods for monitoring cardiac surgical performance and their interpretation. J Thorac Cardiovasc Surg 2004;128:811-9. [Crossref] [PubMed]
- Novick RJ, Fox SA, Stitt LW, et al. Assessing the learning curve in off-pump coronary artery surgery via CUSUM failure analysis. Ann Thorac Surg 2002;73:S358-62. [Crossref] [PubMed]
- Novick RJ, Stitt LW. The learning curve of an academic cardiac surgeon: use of the CUSUM method. J Card Surg 1999;14:312-20; discussion 321-2. [Crossref] [PubMed]
- Novick RJ, Fox SA, Stitt LW, et al. Cumulative sum failure analysis of a policy change from on-pump to off-pump coronary artery bypass grafting. Ann Thorac Surg 2001;72:S1016-21. [Crossref] [PubMed]
- Song MH. A learning curve in aortic dissection surgery with the use of cumulative sum analysis. Nagoya J Med Sci 2014;76:51-7. [PubMed]
- Sihag S, Le B, Witkin AS, et al. Quantifying the learning curve for pulmonary thromboendarterectomy. J Cardiothorac Surg 2017;12:121. [Crossref] [PubMed]
- Tong G, Zhang B, Zhou X, et al. Bilateral versus unilateral antegrade cerebral perfusion in total arch replacement for type A aortic dissection. J Thorac Cardiovasc Surg 2017;154:767-75. [Crossref] [PubMed]
- Shen Y, Liu C, Fang C, et al. Oxygenation impairment after total arch replacement with a stented elephant trunk for type-A dissection. J Thorac Cardiovasc Surg 2018;155:2267-74. [Crossref] [PubMed]
- Matalanis G, Ip S. Total aortic repair for acute type A aortic dissection: a new paradigm. J Vis Surg 2018;4:79. [Crossref] [PubMed]
- Kim JB, Chung CH, Moon DH, et al. Total arch repair versus hemiarch repair in the management of acute DeBakey type I aortic dissection. Eur J Cardiothorac Surg 2011;40:881-7. [PubMed]
- Poon SS, Theologou T, Harrington D, et al. Hemiarch versus total aortic arch replacement in acute type A dissection: a systematic review and meta-analysis. Ann Cardiothorac Surg 2016;5:156-73. [Crossref] [PubMed]
- Di Eusanio M, Berretta P, Cefarelli M, et al. Total Arch Replacement Versus More Conservative Management in Type A Acute Aortic Dissection. Ann Thorac Surg 2015;100:88-94. [Crossref] [PubMed]
- Hsieh WC, Kan CD, Yu HC, et al. Ascending aorta replacement vs. total aortic arch replacement in the treatment of acute type A dissection: a meta-analysis. Eur Rev Med Pharmacol Sci 2019;23:9590-611. [PubMed]
- Lio A, Nicolo F, Bovio E, et al. Total Arch versus Hemiarch Replacement for Type A Acute Aortic Dissection: A Single-Center Experience. Tex Heart Inst J 2016;43:488-95. [Crossref] [PubMed]
- Sun L, Qi R, Zhu J, et al. Total arch replacement combined with stented elephant trunk implantation: a new "standard" therapy for type a dissection involving repair of the aortic arch? Circulation 2011;123:971-8. [Crossref] [PubMed]
- Zhang Q, Ma X, Zhang W, et al. Surgical repair and reconstruction of aortic arch in debakey type I aortic dissection: recent advances and single-center experience in the application of branched stent graft. J Cardiothorac Surg 2017;12:86. [Crossref] [PubMed]


