
Image guided thermal ablation in lung cancer treatment
Introduction
Lung cancer is one of the most common cancers, and also one of the leading cause of cancer death around the world (1). According to the World Health Organization, lung cancer is responsible for an estimated 1.76 million deaths in 2018 worldwide (2).
Radical resection is the first choice for surgical candidates, and has the best outcomes compared with other therapies. However, only 20–30% of patents with lung cancer are operable (3). Other patients have to seek non-surgical treatments, including image guided thermal ablation, SBRT, chemotherapy, radiotherapy, chemoradiotherapy, and immunotherapy.
As one of the local therapies, image guided lung thermal ablation, was first introduced as a lung cancer treatment about two decades ago (4), and represents a collection of different techniques. It is applied in medically inoperative patients with early stage primary lung cancer, and patients with oligometastasis or local recurrence (5,6). Radiofrequency ablation (RFA) and microwave ablation (MWA) are two most widely used thermal ablation for inoperable lung cancer patients. Cryoablation, which induces cell death through extremely low temperature, is now practiced worldwide, and seems promising according to current data. Other techniques, including laser ablation and irreversible electroporation (IRE), are not widely used in lung ablation due to lack of clinical data.
Indications
Image guided thermal ablation has been practiced in many centers all over the world, and has been proven to be cost-effective with good patient experience (6).
The first indication is local therapy for medically inoperable patients with early stage primary lung cancer (5). For surgical candidates, radical surgery is always the best choice. However, for inoperable patients with severe morbidities, poor performance status level, limited lung function, and/or unwillingness to undergo surgery, local treatment and local control of tumor spread needs to be addressed by alternative means. In rare cases, cure can be achieved with image guided thermal ablation. Local recurrence is a major risk of lung ablation. If a patient has local recurrence after ablation, up to 3 repeated ablations is an option in our experience.
The second indication is treatment for multiple lung cancers when definitive local therapy is possible (7). Surgical resection is sometimes impossible because of poor preoperative pulmonary function or predicted massive loss of lung parenchyma. Lung ablation can preserve more normal tissue than surgery, resulting in preservation of post procedural patient quality of life and lung function.
Pulmonary oligometastasis is another indication for imaged guided thermal ablation (8). Lung is the most common target for cancer metastasis compared to other organs. Patient outcomes can be improved if all oligometastasis are removed or inactivated in certain kinds of cancers, for instance, hepatocellular carcinoma and colorectal adenocarcinoma. In the circumstances when surgical resection is not feasible, lung ablation can be a viable alternative.
Local therapy should also be considered when asymptomatic progression is observed in patients with chemo-, radio- or immunotherapy (6). For limited lesions that do not response well to systemic treatment, image guided thermal ablation can be used as definitive local therapy to achieve complete tumor eradication.
However, in order to render a comprehensive and best treatment model for patients, every case should be submitted to multidisciplinary tumor boards with inputs from surgery, interventional and diagnostic radiology, medical and radiation oncology before treatment is applied.
Radio-frequency ablation
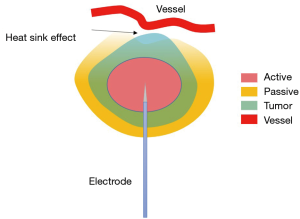
Radio-frequency ablation is one kind of hyperthermal approaches, and was the first applied ablation technique in the lungs. In RFA targeted area, an active electrode oscillates at a frequency of approximately 400 kHz to induce an electrical current which in turn generates heat. Subsequent protein denaturation and coagulation necrosis can be induced under high temperature, which is generally above 55 °C (9). Although only one probe works at a time in RFA, researches of multiple-electrode switching system are ongoing to evaluate its clinical efficacy and safety (10,11). Tissue electrical conductance is an important factor for heat generation. Malignant tissue has higher electrical conductance than normal tissue, thus most electrical current passes through the malignant tissue, resulting in heat accumulation in this area. Malignant tissue also has higher heat conductance than normal tissue, which also helps to trap heat in this area and spare adjacent normal parenchyma. There are several limitations of RFA (12). First, various available devices for RFA operate in a monopolar mode, with grounding pads attached to the skin, allowing for possible skin burns. Feasible solutions include increased surface area of pad, increased number of pads and sequential activation, simultaneous skin temperature monitoring (13). Secondly, electrical and heat conductance is affected by possible charring tissue formed during ablation around the electrode. Improved protocols and devices might help to avoid the rapid rise of temperature and therefore reduce charring. ‘Heat sink’ phenomenon is another problem (Figure 1). Large blood vessels and airways near the ablation zone can drain heat, decreasing the temperature to sublethal threshold and adversely affecting the ablation effectiveness. When vessel is greater than 4 mm, heat sink would be remarkable (14). More intense heating may be a solution for this problem in RFA. However, switching to MWA or cryoablation which is less sensitive to ‘heat sink’ is recommended when treating lesion adjacent to large vessels or airways. Tumor size >3 cm is a predictor of higher recurrence rate in RFA (5). In order to achieve homogeneous necrosis and 1 cm margin, overlapping spheres are required when tumor size is larger than 3 cm in diameter (15). The linear increase of tumor size leads to exponential increase of the number of overlapping spheres. And it results in longer procedure time, higher chance for complications, and higher incidence of leaving behind viable tumor islands (16).
MWA
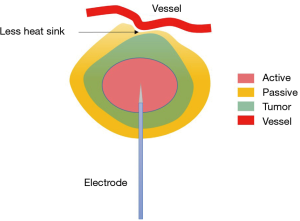
MWA is another kind of hyperthermal approach, using electromagnetic waves in the microwave energy spectrum to generate heat (17). Unlike RFA, electric current and cutaneous grounding pad are not needed, and multiple probes are allowed in MWA. The oscillation of electromagnetic field around the MW antennas induces constant realignment and agitation of water molecules, which leads to increased kinetic energy, ultimately resulting in heat generation. MWA is not restrained by tissue electric and heat conduction, and can reach a larger ablation volume than RFA. It is also less sensitive to the ‘heat sink’ phenomenon due to greater heat generation from microwave energy, larger zone of active heating, and less dependence on thermal conduction than RFA (Figure 2) (18,19). Therefore, MWA can be applied in tumors adjacent to large vessels and airways. The shape of MWA ablation zone is oblate, not spherical. This feature impairs the predictability of the size and shape of the ablation zone.
Cryoablation
Cryoablation has different underlying mechanism inducing cell death compared to RFA and MWA. Pressurized argon gas is distributed through an orifice in the probe to achieve a subzero temperature in Celsius (20). Multiple studies have proved that temperature below −40 °C should be achieved to induce cryogenic destruction and complete cell death (21). Cryoablation is based on the Joule-Thomson effect, which means higher initial gas pressure is related with lower ablation temperature and formation of larger ice balls (22). Several cycles of freezing and thawing can lead to protein denaturation, cell membrane disruption and cell rupture due to osmotic shifts, and tissue ischemia due to microvascular thrombosis. The effects of cryoablation can be affected by many factors, including the number of cycles, duration of procedure, different auxiliary warming devices, tumor location, and regional blood flow. Another possible effect of cryoablation is its pro-immune function. Tumor cell content is not damaged during cryoablation and may be presented to immune cells after cell rupture and therefore triggering an antitumor immune reaction. Such antitumor immune reaction may also help enhance efficacy of subsequent immunotherapy. In addition to cell contents, collagenous architecture is also preserved in cryoablation. This is helpful for tumors locating near the central airways. An additional benefit of MWA is that more than 1 probe can be used at a given cryoablation session, which shortens the procedure time and improves patient experience. Cryoablation induced hypothermic injury is also believed to be better tolerated than hyperthermic injury from RFA and MWA (23).
Comparison among RFA, MWA, and cryoablation
There are very limited data comparing survival and recurrent rates among different contemporary ablation techniques. We herein provide a brief comparison between RFA, MWA, and cryoablation (Table 1) according to current studies.
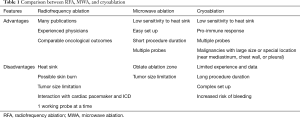
Full table
RFA was first introduced for clinical treatment in 2000 (4), with the longest history and thus the most experienced operators compared to other modalities. One disadvantage is that RFA cannot be utilized when the tumor is located next to large vessels, airway, and other important structures due to potential thermal injury. Other limitations include heat sink phenomenon and rapid charring.
MWA uses a different mechanism to generate heat from RFA, and is less likely to be affected by thermal and electrical impedance. Theoretically, MWA can achieve larger ablation volume and higher temperature than RFA. However, most comparisons of outcomes between MWA and RFA indicate they have similar effects. The disadvantages of RFA do not compromise its oncological outcomes when compared with MWA. Possible reason is their similar mechanism of action, leading to similar outcomes. The randomized controlled trial LUMIRA evaluated the effectiveness of RFA and MWA in lung tumors with 12 months of follow-up (24). It showed no significant differences of overall survival between RFA and MWA. Less intraoperative pain and a significant reduction in tumor mass were observed in the MWA group. Another retrospective, case-controlled observational study enrolled 238 patients, proved similar median progression-free (RFA vs. MWA: 12.5 months, vs. 9.5 months, P=0.673) and overall survival (RFA vs. MWA: 33 months, vs. 30 months, P=0.410) between the RFA and MWA groups (25).
Cryoablation is a relatively new and promising modality. It does not ruin the collagenous architecture of the target area, helps promote immune response by preserving and presenting cancer cell contents. Disadvantages include long procedure duration, increased risk of bleeding, and complex preparation. Moore and colleagues reported a 5-year survival rate of 67.8%±15.3%, cancer-specific survival rate at 5 years of 56.6%±16.5%, 5-year progression-free survival rate of 87.9%±9% (26) in 45 patients with stage I NSCLC after cryoablation. Their results were very encouraging and were comparable to another thermal ablation. Contrarily, a meta-analysis performed by Jiang et al. reported worse outcomes from cryoablation compared to RFA and MWA (27). Therefore, it is still controversial regarding cryoablation’s superiority over RFA and MWA. Randomized controlled trial with large volumes is required.
Other technologies—IRE and laser ablation
IRE is a relatively new ablation technique using pulsed electric fields to induce reversible or irreversible opening of pores along cell membranes which ultimately leads to apoptotic cell death (28,29). Disrupted lipid bilayer by high electrical voltage and destabilized electrical potential on cell membrane are reasons for pore formation. This technique is first used in 2005 in liver ablation by Davalos et al. (30). However, due to poor local control rates in lung cancer demonstrated by the ALICE trial (31), IRE is not recommended in lung ablation.
Laser ablation is another kind of thermal ablation, using catheter delivered laser energy to the target area. However, due to limited available data, its application in lung cancer has not gained much attention.
Trans-bronchial peripheral ablation
Most ablation are performed through percutaneous puncture. Trans-bronchial ablation is a new approach developed in recent years (32). It does not violate the pleural space, so as to avoid certain complications, such as pneumothorax, bronchopleural fistula (BPF), and pleural effusion. RFA and MWA ablation have been applied in this approach. Relevant data is still limited. Several clinical trials are underway, and results should be available soon.
Complications from image guided ablation
Generally, imaged guided thermal ablation has a good safety profile, with very low morbidity and mortality. A national data analysis indicated that in-hospital mortality rate is as low as 1.3% (33). The median hospital stay is reported to be 1 day (33).
Pneumothorax is the most common complication in lung ablation. The incidence can be as high as 40% in lung ablation (33,34). However, only less than 2% of pneumothorax cases were considered as Grade III complication, requiring interventions in Kashima et al.’s study (34). More than half of these pneumothorax patients did not need any treatment. Chest tube placement and surgical intervention are only necessary in a relatively small number of these patients.
BPF is a much more severe complication and could be occasionally observed (35). The incidence of BPF after ablation is reported as 0.4–0.6% (36). The reason is ablation induced tissue necrosis and subsequent sloughing between the pleural space and bronchus. Intractable or recurrent pneumothorax after ablation is an important sign of BPF, which sometimes cannot be diagnosed until even 2 months after ablation (37). Treatment for BPF is challenging, and may not be effective at times. Surgical repair, endoscopic intervention, and pleurodesis are possible choices.
The incidence of hemorrhage is less than 20% (36). Parenchymal hemorrhage and haemothorax are two different forms of hemorrhage. Risk factors include small lesion, lesion located in the basal and middle lung zones, long needle track, traversing vessels in the track, coagulation disorders, and the use of multi-tined electrodes (37). Most cases are self-limiting, and do not require blood transfusion or other interventions. Hemorrhage is much more common in cryoablation than in RFA or MWA (38). A possible reason is the greater number of probes in cryoablation.
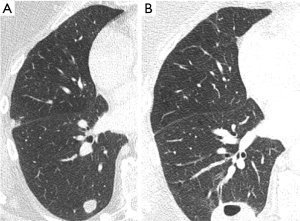
Infectious complications should be treated carefully as deaths from post ablation interstitial pneumonitis or lung abscess have been reported (34). Aseptic pleural effusion may happen in less than 20% of patients, and only require conservative treatment for most of the time. Other rare complications include needle track seeding, thermal injury to nearby organ, pulmonary necrosis resulting in cavitation (Figure 3), nerve injury, rib fracture.
Patient with cardiac pacemaker or implantable cardioverter defibrillator (ICD) should not undergo RFA, because the electrical current and high frequency signals generated by RFA can possibly interact with and disrupt pacing or ICD therapy (39,40). MWA and cryoablation are possible choices in this circumstance.
Ablation versus other local therapy
Lung ablation aims to fully ablate the mass along with adequate margin. However, it is not a curative treatment for lung cancer patients. For patients with early stage primary lung cancer, surgical lobectomy is always the best choice when patients are surgical candidates. For patients who are not willing to or cannot undergo radical surgery, compromised local therapies includes sublobar resection (SLR, wedge resection or segmentectomy), stereotactic body radiation therapy (SBRT), and ablation.
Sublobar resection preserves more lung parenchyma than lobectomy, and is believed to have better outcomes than SBRT or ablation. A propensity matched analysis enrolled 53,973 stage I NSCLC patients from the National Cancer Database to compare the long-term outcome of SLR, SBRT, and ablation (41). After propensity match, a significant difference in overall survival was observed in the SLR group compared to the SBRT or ablation groups.
Data comparing survival outcomes between SBRT and ablation is diverse because of the heterogeneity on study groups and protocols. Bilal et al. reviewed 16 related studies, and indicated that SBRT offers a higher 5-year survival rate (47% versus 20.1–27%) and lower local progression rate (3.5–14.5% vs. 23.7–43%) than ablation (42). They also found that larger tumor is related to worse outcome in ablation, suggesting 3 cm as the cut off diameter for ablation. Another study analyzed data from Surveillance, Epidemiology, and End Results database, including stage IA lung cancer patient data from 2004 to 2015, concluded that there is no significant differences of overall survival between SBRT and ablation (43). A prospective clinical trial (ACOSOG Z4033) reported 86.3% overall survival rate at 1 year and 69.8% at 2 years for stage IA patients who had undergone RFA (44). Local recurrence rate was 68.9% at 1 year and 59.8% at 2 years, and worse for tumors >2 cm than tumors ≤2 cm. Their data is comparable to data reported in SBRT studies with similar patients. Currently, National Comprehensive Cancer Network guidelines recommend that SBRT should be first considered because of its possible better outcome, and ablation is appropriate for medically inoperable patients when local control is not considered as the highest priority (7). Randomized controlled study with large volume is still necessary to reach a clear conclusion.
Post ablation surveillance
Follow-up is commonly required after lung ablation. The purpose is to find early signs of local recurrence and tumor progression. Most patients are being surveilled every 3 months in the first year, followed by every 6 months thereafter. Individualized plan may vary due to different tumor type and stage. Multidisciplinary tumor board determines the exact time interval and time span for each patient.
Contrast enhanced computed tomography (CT), and positron emission tomography – computed tomography (PET-CT) are viable surveillance choices. High resolution CT provides morphologic information regarding post ablation parenchymal changes, local tumor growth or recurrence as well as metastatic status. Intravenous contrast provides further information regarding tumor perfusion. PET-CT is recommended by many experts because it provides metabolic information not gleaned from CT. However, one must be cognizant that the CT performed along with PET is limited in resolution and is suboptimal for morphologic evaluation. Baseline and one-month post ablation follow-up PET imaging (to establish new baseline) is typically obtained.
One needs to be aware of the slow morphologic evolution of the ablation zone, potentially resulting in false positive results for tumor recurrence during follow-up. Five post ablation CT patterns have been describe by Palussière et al. (45): fibrosis, cavitation, nodule, atelectasis, and disappearance. It is imperative to distinguish these normal evolutions from local disease progression as subsequent management differs between these situations.
Limitations
There are several limitations of image guided thermal ablation. Local recurrence is a major concern of lung ablation compared to surgery. Moreover, ablation is limited to regional disease control, while SBRT or segmentectomy can eliminate metastases such as nodal disease concomitantly. Tumor size and location may affect the outcome of ablation, especially in RFA. ‘Heat sink’ phenomenon can affect the outcome for tumors adjacent to large vessels or airways. Repeated punctures are required in large tumors.
Conclusions
Image guided thermal ablation has been proven to be a safe and feasible alternative to surgery with acceptable morbidity and mortality rate in medically inoperable lung cancer patients. As a minimally invasive technique, lung ablation has several advantages over surgery, including high local control rates, shorter in-hospital stay, comparatively lower cost and better patient tolerance. RFA, MWA, and cryoablation are currently acceptable techniques for lung ablation. Continue technological advances are bringing forth more ablation methods, including laser ablation and IRE. Large randomized controlled trials remain necessary to further validate their application in lung cancer.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Chi Wan Koo) for the series “Contemporary Practice in Thoracic Neoplasm Diagnosis, Evaluation and Treatment” published in Journal of Thoracic Disease. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/jtd-2019-cptn-08). The series “Contemporary Practice in Thoracic Neoplasm Diagnosis, Evaluation and Treatment” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Siegel RL, Jemal A. Lung Cancer Statistics. Adv Exp Med Biol 2016;893:1-19. [Crossref] [PubMed]
- Organization; WH. Cancer. 2018, September 12. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer
- Mazzone P. Preoperative evaluation of the lung resection candidate. Cleve Clin J Med 2012;79:e17-e22. [Crossref] [PubMed]
- Dupuy DE, Zagoria RJ, Akerley W, et al. Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol 2000;174:57-9. [Crossref] [PubMed]
- de Baere T, Tselikas L, Catena V, et al. Percutaneous thermal ablation of primary lung cancer. Diagn Interv Imaging 2016;97:1019-24. [Crossref] [PubMed]
- Prud'homme C, Deschamps F, Moulin B, et al. Image-guided lung metastasis ablation: a literature review. Int J Hyperthermia 2019;36:37-45. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aggarwal C, et al. Non-small cell lung cancer, version 1.2020: Featured updates to the NCCN guidelines. JNCCN 2019;17:1464-72. [PubMed]
- Palussière J, Catena V, Buy X. Percutaneous thermal ablation of lung tumors–Radiofrequency, microwave and cryotherapy: Where are we going? Diagn Interv Imaging 2017;98:619-25. [Crossref] [PubMed]
- Hong K, Georgiades C. Radiofrequency ablation: mechanism of action and devices. J Vasc Interv Radiol 2010;21:S179-86. [Crossref] [PubMed]
- Kodama H, Yamakado K, Hasegawa T, et al. Radiofrequency Ablation Using a Multiple-Electrode Switching System for Lung Tumors with 2.0-5.0-cm Maximum Diameter: Phase II Clinical Study. Radiology 2015;277:895-902. [Crossref] [PubMed]
- Chang W, Lee JM, Lee DH, et al. Comparison of switching bipolar ablation with multiple cooled wet electrodes and switching monopolar ablation with separable clustered electrode in treatment of small hepatocellular carcinoma: A randomized controlled trial. PLoS One 2018;13:e0192173. [Crossref] [PubMed]
- Tafti BA, Genshaft S, Suh R, et al. Lung Ablation: Indications and Techniques. Semin Intervent Radiol 2019;36:163-75. [Crossref] [PubMed]
- Huffman SD, Huffman NP, Lewandowski RJ, et al. Radiofrequency ablation complicated by skin burn. Semin Intervent Radiol 2011;28:179-82. [Crossref] [PubMed]
- Lu DS, Raman SS, Vodopich DJ, et al. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the "heat sink" effect. AJR Am J Roentgenol 2002;178:47-51. [Crossref] [PubMed]
- Dodd GD 3rd, Frank MS, Aribandi M, et al. Radiofrequency thermal ablation: computer analysis of the size of the thermal injury created by overlapping ablations. AJR Am J Roentgenol 2001;177:777-82. [Crossref] [PubMed]
- Rose SC, Thistlethwaite PA, Sewell PE, et al. Lung cancer and radiofrequency ablation. J Vasc Interv Radiol 2006;17:927-51. [Crossref] [PubMed]
- Lubner MG, Brace CL, Hinshaw JL, et al. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol 2010;21:S192-S203. [Crossref] [PubMed]
- Brace CL, Laeseke PF, Sampson LA, et al. Microwave ablation with a single small-gauge triaxial antenna: in vivo porcine liver model. Radiology 2007;242:435-40. [Crossref] [PubMed]
- Wright AS, Sampson LA, Warner TF, et al. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology 2005;236:132-9. [Crossref] [PubMed]
- Erinjeri JP, Clark TW. Cryoablation: mechanism of action and devices. J Vasc Interv Radiol 2010;21:S187-91. [Crossref] [PubMed]
- Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology 1998;37:171-86. [Crossref] [PubMed]
- Sprenkle PC, Mirabile G, Durak E, et al. The effect of argon gas pressure on ice ball size and rate of formation. J Endourol 2010;24:1503-7. [Crossref] [PubMed]
- Allaf ME, Varkarakis IM, Bhayani SB, et al. Pain control requirements for percutaneous ablation of renal tumors: cryoablation versus radiofrequency ablation—initial observations. Radiology 2005;237:366-70. [Crossref] [PubMed]
- Macchi M, Belfiore MP, Floridi C, et al. Radiofrequency versus microwave ablation for treatment of the lung tumours: LUMIRA (lung microwave radiofrequency) randomized trial. Med Oncol 2017;34:96. [Crossref] [PubMed]
- Chi J, Ding M, Shi Y, et al. Comparison study of computed tomography-guided radiofrequency and microwave ablation for pulmonary tumors: A retrospective, case-controlled observational study. Thorac Cancer 2018;9:1241-8. [Crossref] [PubMed]
- Moore W, Talati R, Bhattacharji P, et al. Five-year survival after cryoablation of stage I non-small cell lung cancer in medically inoperable patients. J Vasc Interv Radiol 2015;26:312-9. [Crossref] [PubMed]
- Jiang B, Mcclure MA, Chen T, et al. Efficacy and safety of thermal ablation of lung malignancies: A Network meta-analysis. Ann Thorac Med 2018;13:243-50. [Crossref] [PubMed]
- Neu WK, Neu JC. Mechanism of irreversible electroporation in cells: Insight from the models. In: Rubinsky B. Irreversible Electroporation. Berlin: Springer, 2010:85-122.
- Miller L, Leor J, Rubinsky B. Cancer cells ablation with irreversible electroporation. Technol Cancer Res Treat 2005;4:699-705. [Crossref] [PubMed]
- Davalos RV, Mir IL, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng 2005;33:223-31. [Crossref] [PubMed]
- Ricke J, Jürgens JH, Deschamps F, et al. Irreversible electroporation (IRE) fails to demonstrate efficacy in a prospective multicenter phase II trial on lung malignancies: the ALICE trial. Cardiovasc Intervent Radiol 2015;38:401-8. [Crossref] [PubMed]
- Sabath BF, Casal RF. Bronchoscopic ablation of peripheral lung tumors. J Thorac Dis 2019;11:2628-38. [Crossref] [PubMed]
- Welch BT, Brinjikji W, Schmit GD, et al. A national analysis of the complications, cost, and mortality of percutaneous lung ablation. J Vasc Interv Radiol 2015;26:787-91. [Crossref] [PubMed]
- Kashima M, Yamakado K, Takaki H, et al. Complications after 1000 lung radiofrequency ablation sessions in 420 patients: a single center's experiences. AJR Am J Roentgenol 2011;197:W576-80. [Crossref] [PubMed]
- Zheng A, Yang X, Ye X, et al. Bronchopleural fistula after lung ablation: Experience in two cases and literature review. Indian J Cancer 2015;52 Suppl 2:e41-6. [Crossref] [PubMed]
- Hiraki T, Gobara H, Fujiwara H, et al. Lung cancer ablation: complications. Seminars in interventional radiology; 2013: Thieme Medical Publishers.
- Cannella M, Cornelis F, Descat E, et al. Bronchopleural fistula after radiofrequency ablation of lung tumours. Cardiovasc Intervent Radiol 2011;34 Suppl 2:S171-4. [Crossref] [PubMed]
- Inoue M, Nakatsuka S, Yashiro H, et al. Percutaneous cryoablation of lung tumors: feasibility and safety. J Vasc Interv Radiol 2012;23:295-302. [Crossref] [PubMed]
- Donohoo JH, Anderson MT, Mayo-Smith WW. Pacemaker reprogramming after radiofrequency ablation of a lung neoplasm. AJR Am J Roentgenol 2007;189:890-2. [Crossref] [PubMed]
- Fiek M, Dorwarth U, Durchlaub I, et al. Application of radiofrequency energy in surgical and interventional procedures: are there interactions with ICDs? Pacing Clin Electrophysiol 2004;27:293-8. [Crossref] [PubMed]
- Wu J, Bai HX, Chan L, et al. Sublobar resection compared with stereotactic body radiation therapy and ablation for early stage non–small cell lung cancer: A National Cancer Data Base study. J Thorac Cardiovasc Surg 2020;160:1350-7.e11. [Crossref] [PubMed]
- Bilal H, Mahmood S, Rajashanker B, et al. Is radiofrequency ablation more effective than stereotactic ablative radiotherapy in patients with early stage medically inoperable non-small cell lung cancer? Interact Cardiovasc Thorac Surg 2012;15:258-65. [Crossref] [PubMed]
- Liang L, Li G, Xie S, et al. Choice of Treatment for Stage IA Non-small Cell Lung Cancer Patients Ineligible for Surgery: Ablation or Stereotactic Body Radiotherapy? J Cancer 2020;11:1634-40. [Crossref] [PubMed]
- Dupuy DE, Fernando HC, Hillman S, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in medically inoperable patients: Results from the American College of Surgeons Oncology Group Z4033 (Alliance) trial. Cancer 2015;121:3491-8. [Crossref] [PubMed]
- Palussière J, Marcet B, Descat E, et al. Lung tumors treated with percutaneous radiofrequency ablation: computed tomography imaging follow-up. Cardiovasc Intervent Radiol 2011;34:989-97. [Crossref] [PubMed]


