Surgical specimen histology revealed inadequacy of conventional transbronchial needle aspiration sample in the diagnosis of adenosquamous lung carcinoma
Introduction
Transbronchial needle aspiration (TBNA) is an established minimally invasive procedure used in the diagnosis and staging of bronchogenic carcinoma (1). In addition, it is also used in the diagnosis of benign mediastinal or hilar located lesions and enlarged lymph nodes (1). In recent years, it has been considered as conventional TBNA (cTBNA) since the widely use of endobronchial ultrasound guided TBNA (EBUS-TBNA). Both cTBNA and EBUS-TBNA have good diagnostic yield for central airway located lung cancers, but relatively lower accuracy for benign diseases (2).
In most studies, the diagnostic performance of cTBNA or EBUS-TBNA was based on the histological and/or cytological diagnosis of TBNA samples, and few studies compared the histological diagnosis of TBNA and that of surgery specimens. Due to the minimal amount of TBNA samples, inaccuracy of the histological diagnosis will be unavoidable. Many studies had proved the adequacy of cTBNA or EBUS-TBNA samples for the test of EGFR or other mutations, but whether TBNA samples are as good as surgery samples in the classification of lung cancer is unknown.
We established cTBNA procedure in our departments from October 2012. Here we retrospectively reviewed the diagnostic performance of cTBNA in patients with lesions located in or around the central airways identified by CT scan, and compared the histological diagnosis of central airway lesions from patients who underwent cTBNA and subsequent surgery.
Methods
Patients
Between October 2012 and April 2014, 63 patients with identified central airway lesions (trachea, left and right main bronchi, mediastinal or hilar lesions) underwent cTBNA at the bronchoscope suites of Wuhan No. 1 Hospital, China. The study was approved by the institutional review board of Wuhan No. 1 Hospital. A total of 45 patients diagnosed with TBNA specimens as lung cancer or atypical neoplasia, and 23 of them received surgical treatment.
TBNA procedure
cTBNA was conducted under flexible bronchoscope (BF-1t260, Olympus, Tokyo, Japan). All patients received contrast-enhanced CT scan prior to the procedure in order to get the information about the precise location of the lesions. The patients with lesions located in central airways, hilar or mediastinal masses or enlarged lymph nodes were indicated for cTBNA and included in the present study. All patients were screened for total platelet counting, electrocardiography and coagulation function test. Patients in whom the procedure was contradicted were excluded from the study. The procedure was conducted under local anesthesia with 2% lidocaine and conscious sedation with midazolam and fentanyl. The puncture sites were determined according to the CT-scan as suggested by Wang et al. (3). The distal tip of bronchoscope was fixed near the sites. The 19-gauge aspirating needle (WANG Transbronchial Histology Needle, MW-319) was pushed out from the distal tip of bronchoscope and puncture into the lesions for 0.5-1 cm. Two to four aspirates were performed with ten passes (moving needle back and forth in the lesions) per aspirate. The samples were expelled out of the needle. The fragments of tissue were collected for pathological examination, the cellular debris were flushed out of the needle and smeared onto glass slides, air dried, fixed with 95% alcohol, and illustrated with papanicolaou staining. Tissue fragments were fixed with 10% neutral buffered formalin and stained with hematoxylin and eosin (H&E). When necessary, immunohistochemistry staining was performed to classifying the lung cancers. We could not perform rapid on site evaluation (ROSE).
Histology
The surgery resected specimens were formalin-fixed; the representative tissue was cut into 1 cm × 1 cm × 0.5 cm thick blocks including tumor tissue and an edge of surrounding normal lung or bronchial tissue, and then parafilm embedded. The tissue slides were routinely H&E stained. The tissue slides were then used for immunohistochemistry assay with different antibodies. The antibodies used for adenocarcinoma (ADC) were thyroid transcriptional factor-1 (TTF-1, Abcam, Cambridge, CA, USA) and Napsin A (Abcam, Cambridge, CA, USA), the antibodies for squamous cell carcinoma (SCC) was CK5/6 (cytokeratin 5/6, Santa Cruz, Dallas, TX, USA) and P63 (Santa Cruz, Dallas, TX, USA).
Statistics
Descriptive statistics are presented. The diagnostic accuracy, sensitivity, specificity, positive predictive value and negative predictive value of cTBNA were calculated according to the standard definition.
Results
Sixty-three patients (55 males and 8 females) with mean age of 59.2±10.4 years underwent cTBNA using 19 gauge needles. According to cytological and/or histological findings, the diagnostic yield was 84.9% (45/53) for suspected malignancy and 60.0% (6/10) for suspected benign diseases. The total diagnostic yield was 80.9% (51/63) (Table 1).
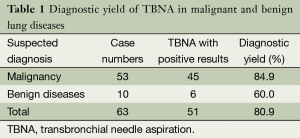
Full table
A total of 45 patients were diagnosed as lung cancer or atypical hyperplasia according to TBNA cytology and/or histology. Among them 23 patients underwent surgery, including lobectomy, pneumonectomy or sleeve resection. The final histological types of these lesions were summarized in Table 2.
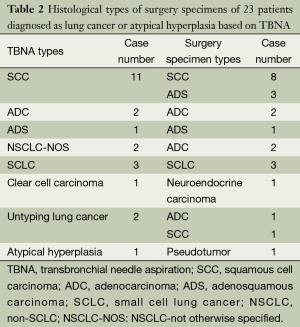
Full table
We compared the performance of cTBNA in subtyping of lung cancers with that of surgery specimen. The overall diagnostic yield of cTBNA for lung cancer was 95.7% (22/23), but the accuracy for histological classification of lung cancer is only 63.6% (14/22), for adenosquamous carcinoma (ADS) was only 25% (1/4).
Figure 1 shows a typical lesion diagnosed as squamous cell cancer based on TBNA samples and finally diagnosed as ADS according to histopathology of surgery specimen. Figure 2 shows the images of H&E and immunohistochemistry staining of TBNA specimens from the patient diagnosed as ADS based on TBNA specimens. There were no major complications observed during cTBNA. A few patients had mild bleeding, which was controlled by local treatment.
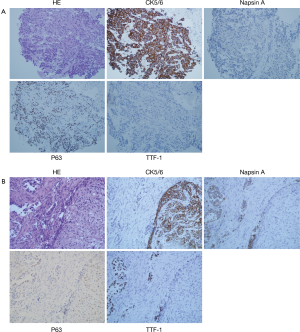
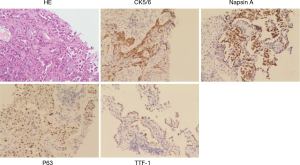
Discussion
In the present study, we presented data to show that cTBNA under flexible bronchoscopy is a safe procedure in the diagnosis of central airway located lesions, but the accuracy of cTBNA in the subtyping of lung cancer is relatively low, especially for lung ADS.
In our study, the total diagnostic yield of cTBNA was 80.9%, which is similar to that reported by Khoo et al. (4). They gave a yield of 65% for evaluation of mediastinal disease, both benign and malignant. Non-small cell lung cancer (NSCLC) was the most common malignant disease in our series. We note that there is a relative high diagnostic yield of small cell lung cancer (SCLC) in our series. This is consistent with the study by Hsu et al., who reported higher yields for patients featuring SCLC (5).
A few factors have been found to influence the diagnostic yields of cTBNA. A recent system review by Bonifazi et al. summarized that the major predictors that influence the accuracy of cTBNA include unselected population or patients suspected of lung cancers, lymph node size, abnormal endoscopic findings, right paratracheal or subcarinal locations and the use of histological needles (6).
The prevalence of lung cancer is the most important factor that influences the diagnostic yield of cTBNA. As shown in our series and many other studies, the diagnostic yield of malignancy is much higher than benign diseases (2). A recent study by Rakha et al. assessed the cytological outcome of cTBNA and found that cTBNA had a relatively higher sensitivity for the diagnosis of malignant lesions (7). Because of the lower diagnostic yield of benign disease by cTBNA, only ten patients suspected of benign diseases were included in our series.
Hermens et al. showed that in a group of pulmonologists experienced with bronchoscopy, the diagnostic yield of cTBNA was 77% at start and increased to 82% after 32 months of experience (8), thus there was no learning curve for this technique. The diagnostic yield was related to lymph node size, but not the location (8). Hsu et al. also observed an improvement in the performance of cTBNA over time (5). When the lymph node size was >20 mm, the learning curve does not exist. We did not find any learning curve in our study since the operator was trained in an interventional pulmonology center for 3 months prior to the start of cTBNA.
In order to increase the diagnostic yield of cTBNA, CT fluoroscopy guidance was used to confirm the location of the needles (9). We did not adopt CT fluoroscopy guidance in our cTBNA procedure since it was unavailable in our suite.
ROSE of cytological specimen smears is a technique to assess the adequacy of TBNA specimens (10). It requires a cytopathologist and other resources; therefore we could not accomplish ROSE in our series due to the unavailability of these resources.
Most of previous studies focused on the assessment of the diagnostic yield of cTBNA, and the performance of pathohistological classification of cTBNA was based on the histology or cytology of cTBNA samples, less is based on surgery specimens. In the present study, we compared the pathohistological classification of lung cancer patients who underwent both cTBNA and surgery. Although the total diagnostic yield of cTBNA is 80.9% in our series, TBNA is not very accurate in pathhistological classification of lung cancer, since the accuracy of cTBNA for histological classification of lung cancer is only 60.6%. We found that cTBNA had an excellent performance in differentiation between SCLC and NSCLC.
It is noticeable that in our series there were four cases of ADS lung cancer diagnosed based on pathohistology of surgery specimens, among them only one patient was previously correctly classified by cTBNA pathohistology, the other three patients were initially diagnosed as pure SCC based on cTBNA samples. Similar cases have been reported by other centers in the EBUS-TBNA samples. ADS is defined as tumor comprising with at least 10% malignant ADC and SCC component. These ADC or SCC components will be missed during the biopsy procedure such as cTBNA, especially when they are not uniformed distributed (as shown in Figure 2) or the predominance of a single histology exists. It is necessary to further investigate the clinical features of ADS by involving large amounts of cases and find out which kind of patients have higher probability to be ADS. How to increase the diagnostic accuracy of ADS by cTBNA or EBUS-TBNA should also be investigated. Whether more aspirates or more passes per aspirate of TBNA will increase the possibility to find ADC or SCC components of ADS is still unclear.
Since ADS is less common in NSCLC, few studies have been performed to characterize the genetic and pathological patterns of this subtype of lung cancer. A recent study by Bastide et al. showed that ADS is considered as a mix of ADC and SCC, however there were specific genes discriminating ADS-ADC and ADS-SCC (11). Shiozawa et al. analyzed 67 cases of ADS and found that ADS is more like to occur in older patients than ADC, but less likely to occur in smokers than SCC, no significant correlation of sex was found (12). In 59 patients of ADS who were followed up, the postoperative 3-year survival rate was 58.7%, which is lower than that of ADC (78.1%) (12); in these patients, the frequency of EGFR mutation (24%) was similar to ADC, and mutation positive patients had better survival rate than mutation negative patients (90.0% vs. 62.1%) (12). This is similar to the study by Powrózek et al. and Morodomi et al., which reported a EGFR activating mutation of 28.6% (4/14) and 21.9% (7/32) respectively in ADS patients (13,14). Dragnev et al. reported a case of ADS initially diagnosed as SCC which had ADC component and ALK rearrangement (15). Another study including 76 Chinese cases of ADS investigated known driver mutations and found 56.6% patients had mutation kinases, similar to that of poor differentiated ADC (16), suggesting the importance of recognizing the ADC component of ADS.
The clinical importance of the detection of ADC component from ADS is addressed by recent studies. Song et al. recently reported 49 ADS patients who received erlotinib or gefitinib therapy. The median PFS and OS were 4.3 and 17.6 months respectively, which are not different from ADC patients who received EFGR-TKI as first line therapy (17). Seven patients with EGFR mutation was detected in 21 patients with adequate specimens for EGFR mutation analysis. Five had PR, one had SD, and one had PD in these patients. The PFS of EGFR mutation positive patients and wild type patients were 9.4 and 2.4 months respectively (17). A previous study reported three ADS patients with EGFR mutation received gefitinib therapy, two had SD and one had PR (18). Based on these data, we could conclude that ADS patients with EGFR mutation will benefit from EGFR-TKI treatment.
Fewer cases of EML4-ALK rearrangement have been reported in ADS specimens. The clinical importance of anti-ALK treatment in ADS is still not investigated due to the lower percentages of ALK rearrangement and fewer cases of ADS.
Conclusions
In conclusion, our results demonstrated that cTBNA is a safe and effective technique in the diagnosis of hilar and mediastinal lesions. But, cTBNA specimens had relative lower diagnosis accuracy for NSCLC classification, especially for ADS.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Medford AR, Agrawal S, Free CM, et al. A prospective study of conventional transbronchial needle aspiration: performance and cost utility. Respiration 2010;79:482-9. [PubMed]
- Kupeli E, Memis L, Ozdemirel TS, et al. Transbronchial needle aspiration “by the books”. Ann Thorac Med 2011;6:85-90. [PubMed]
- Wang KP, Mehta AC, Francis Turner JJ. eds. Flexible Bronchoscopy, 2nd Edition. Cambridge, MA: Blackwell Publishing, 2004.
- Khoo KL, Chua GS, Mukhopadhyay A, et al. Transbronchial needle aspiration: initial experience in routine diagnostic bronchoscopy. Respir Med 2003;97:1200-4. [PubMed]
- Hsu LH, Liu CC, Ko JS, et al. Education and experience improve the performance of transbronchial needle aspiration: a learning curve at a cancer center. Chest 2004;125:532-40. [PubMed]
- Bonifazi M, Zuccatosta L, Trisolini R, et al. Transbronchial needle aspiration: a systematic review on predictors of a successful aspirate. Respiration 2013;86:123-34. [PubMed]
- Rakha EA, Naik V, Chaudry Z, et al. Cytological assessment of conventional transbronchial fine needle aspiration of lymph nodes. Cytopathology 2010;21:27-34. [PubMed]
- Hermens FH, Limonard GJ, Termeer R, et al. Learning curve of conventional transbronchial needle aspiration in pulmonologists experienced in bronchoscopy. Respiration 2008;75:189-92. [PubMed]
- Garpestad E, Goldberg S, Herth F, et al. CT fluoroscopy guidance for transbronchial needle aspiration: an experience in 35 patients. Chest 2001;119:329-32. [PubMed]
- Joseph M, Jones T, Lutterbie Y, et al. Rapid on-site pathologic evaluation does not increase the efficacy of endobronchial ultrasonographic biopsy for mediastinal staging. Ann Thorac Surg 2013;96:403-10. [PubMed]
- Bastide K, Ugolin N, Levalois C, et al. Are adenosquamous lung carcinomas a simple mix of adenocarcinomas and squamous cell carcinomas, or more complex at the molecular level? Lung Cancer 2010;68:1-9. [PubMed]
- Shiozawa T, Ishii G, Goto K, et al. Clinicopathological characteristics of EGFR mutated adenosquamous carcinoma of the lung. Pathol Int 2013;63:77-84. [PubMed]
- Powrózek T, Krawczyk P, Ramlau R, et al. EGFR gene mutations in patients with adenosquamous lung carcinoma. Asia Pac J Clin Oncol 2014;10:340-5. [PubMed]
- Morodomi Y, Okamoto T, Takenoyama M, et al. Clinical Significance of Detecting Somatic Gene Mutations in Surgically Resected Adenosquamous Cell Carcinoma of the Lung in Japanese Patients. Ann Surg Oncol 2014; [PubMed]
- Dragnev KH, Gehr G, Memoli VA, et al. ALK-rearranged adenosquamous lung cancer presenting as squamous cell carcinoma: a potential challenge to histologic type triaging of NSCLC biopsies for molecular studies. Clin Lung Cancer 2014;15:e37-40. [PubMed]
- Wang R, Pan Y, Li C, et al. Analysis of major known driver mutations and prognosis in resected adenosquamous lung carcinomas. J Thorac Oncol 2013;9:760-8. [PubMed]
- Song Z, Lin B, Shao L, et al. Therapeutic efficacy of gefitinib and erlotinib in patients with advanced lung adenosquamous carcinoma. J Chin Med Assoc 2013;76:481-5. [PubMed]
- Shukuya T, Takahashi T, Kaira R, et al. Efficacy of gefitinib for non-adenocarcinoma non-small-cell lung cancer patients harboring epidermal growth factor receptor mutations: a pooled analysis of published reports. Cancer Sci 2011;102:1032-7. [PubMed]


