Comparison of diagnostic performances among bronchoscopic sampling techniques in the diagnosis of peripheral pulmonary lesions
Introduction
Peripheral pulmonary lesions (PPLs) are common problems in clinical practice. Clinical data and radiographic finding, such as chest radiography and computed tomography (CT) can provide some clues for diagnosis. However, in some circumstances, definite diagnosis is required before deciding on the appropriate treatment. Therefore, respiratory specimens are needed to identify the etiology of the lesions. Flexible bronchoscopy (FB) is a widely used, minimally invasive diagnostic technique for sampling respiratory specimens. Nevertheless, it is still a challenging task for pulmonologists to obtain accurate samplings that represent the etiologies of PPLs using this approach. FB can reach into the airway up to the subsegmental bronchi; beyond the visual range, the airway continually divides into many generations before the peripheral target is reached. Without guidance, FB cannot guarantee an accurate sampling at the exact location of the PPL, as is shown by its low diagnostic yield (1).
FB under fluoroscopic guidance has been implemented since the 1970s. Its overall diagnostic yield was around 78% (2). Nevertheless, the diagnostic efficacy of this approach is affected by the size of the lesion. For PPLs less than 2 cm, this technique provides a rather low diagnostic yield, varying from 11-42% (2-4).
Radial endobronchial ultrasound (R-EBUS) has been developed to enhance the diagnostic yield of PPLs by providing a detail ultrasound image of the lesion. This can confirm that the instrument has reached the target. In addition, R-EBUS generates more informative images to help select the most promising area for sampling, while fluoroscopy provides only the anatomical location. Compare with fluoroscopy-guided FB, bronchoscopy using R-EBUS with fluoroscopy guidance provided significantly higher diagnostic performance, especially for PPLs smaller than 20 mm (5,6).
There are many sampling techniques associated with R-EBUS-guided FB such as bronchoalveolar lavage (BAL), brushing, and biopsy. However, data regarding the diagnostic performances among bronchoscopic sampling techniques is limited. The aim of this study was to compare the diagnostic yields among bronchoscopic sampling techniques in the diagnosis of PPLs.
Methods
A prospective study was conducted on 112 patients who were diagnosed with PPLs and underwent R-EBUS-guided bronchoscopy between October 2012 and September 2014 in Ramathibodi Hospital, Mahidol University, Thailand. A PPL was defined as a pulmonary lesion that was surrounded by pulmonary parenchyma and was not endoscopically visible by bronchoscopy. A CT scan of the chest was performed in all cases, and the sub-subsegmental bronchus feeding to the PPL was selected prior to the bronchoscopic procedure. Only patients who had demonstrated feeding bronchi, regardless of the size, were selected for R-EBUS- guided FB. The size of the lesion was recorded by its longest diameter on the CT scan. All patients provided written, informed consent prior to participation. The study protocol was approved by the Ethics Committee on Human Experimentation at Ramathibodi Hospital, Faculty of Medicine, Mahidol University.
All bronchoscopies were performed in the bronchoscopy room, equipped with a fluoroscopy unit, using a flexible bronchoscope (BF-P180, Olympus, Tokyo, Japan: external diameter, 4.9 mm; channel diameter, 2.0 mm) via the transnasal route under local anesthesia. The bronchial tree in both lungs was examined down to the level of subsegmental bronchi. Subsequently, a radial ultrasound miniprobe (UM-S20-17S, Olympus, Tokyo, Japan) with a guide sheath (GS) (SG-200C, Olympus, Tokyo, Japan) was inserted through the working channel of the bronchoscope and was advanced to the target sub-subsegmental bronchus until the lesion was located by EBUS and fluoroscopy images. The R-EBUS probe was then moved within the lesion under fluoroscopic control to identify the most suspicious area, in order to obtain the tissue specimens (7,8). Next, the probe was withdrawn under fluoroscopy, leaving the GS in place. A biopsy forceps and a bronchial brush were introduced through the GS to obtain samples from the site marked by fluoroscopy. Five brushings for cytological smear, five brushings for histology, and five biopsy samplings were performed. Finally, the GS was removed and BAL was performed at the target subsegmental bronchus by instilling with 50 mL of normal saline and aspiration into a trap. Either repeated instilling of 50 mL normal saline, filled up to a total of 100-150 mL or receiving 50 mL retrieved fluid indicated adequate lavage.
Specimens from transbronchial biopsy (TBB) were processed as a cell block for pathologic evaluation. They were collected in a microtube filled with 0.5 mL of normal saline and 1 mL of 10% formalin. The samples were centrifuged at 6,000 rpm for 5 min. The pellet was then embedded in paraffin and sectioned. Routine hematoxylin and eosin (H&E) staining was used on all cell block sections. Microbiological and immunohistochemical stains were applied in cases where special staining was indicated. Cell blocks from bronchial brushing samples were also processed in the same way as TBB specimens after the bronchial brush was rinsed in a microtube.
Each bronchial brushing cytology specimen was prepared by smearing on a microscopy slide and fixing immediately in a flask containing 95% alcohol, followed by staining using the Papanicolaou method. The rest of bronchial brushing was then irrigated with 3 mL of sterile normal saline to obtain new sample as rinsed brushing fluid. In addition, 1 mL of sterile normal saline solution was used to flush the material left in the GS into the rinsed brushing fluid sample. This fluid was sent to the laboratory for centrifugation and staining using the Papanicolaou method. The BAL fluid (BALF) was processed for cytology, Gram, acid-fast bacilli and modified Giemsa staining, and also microbial culture in all cases.
All sampling techniques (Figure 1), included TBB, brushing cell block, brushing smear, rinsed fluid of brushing, and BAL, were evaluated for the diagnosis. A definite diagnosis was established when histological or cytological results were defined as malignant disease or specific non-neoplastic disease. Histological or cytological diagnosis of non-specific inflammation was considered to be non-diagnostic, although the final diagnosis proved to be a benign process. The result of BALF culture was not considered for the diagnostic yield, even if it ultimately demonstrated the pathogen relevant to the final diagnosis.

Statistical analysis
All data were analyzed with a statistical software package (SPSS for Windows version 16.0; SPSS, Chicago, IL, USA). Values were expressed as mean ± standard deviation for continuous variables, and as frequencies and percentages for categorical variables. The effect of size and the etiology of PPLs on the diagnostic yield of each sampling technique were analyzed using a chi-square (χ2) test. The diagnostic yields of each sampling technique were compared using Cochran’s Q test. If statistical significance was reached, the McNemar test was then performed to compare each technique with that achieving the highest diagnostic yield. All statistical tests were two-sided; P<0.05 was considered to be statistically significant.
Results
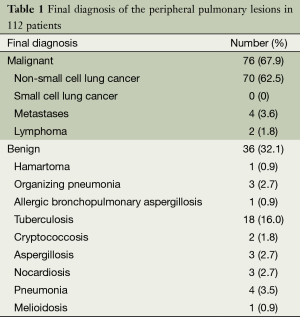
There were 112 patients comprising 57 males and 55 females, with an average age of 58.2±14.2 years. These included 76 malignancies and 36 benign lesions (Table 1). The mean diameter of the PPLs was 23.5±9.5 mm. Although only patients who had demonstrated feeding bronchi were selected, the R-EBUS probe could not locate the lesion in two cases. For 92 of the lesions, the probe could advance to within the lesion on the EBUS image, while in the other 18 cases; the probe was adjacent to the lesion.
Full table
The overall diagnostic yield of EBUS-guided bronchoscopy was 80.4% (95% CI: 72.9-87.8%). Of the 22 patients with nondiagnostic R-EBUS-guided bronchoscopy, the final diagnoses and diagnostic methods are shown in Table 2. The final diagnoses were established on clinical grounds in four infectious diseases (one tuberculosis, two pneumonias, and one melioidosis) and one immunologic process (allergic bronchopulmonary aspergillosis). All of these PPLs improved after specific treatment.

Full table
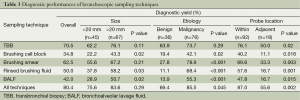
Table 3 shows the diagnostic yield of different bronchoscopic sampling techniques. Etiology of the PPLs was a significant factor for the diagnostic performance of EBUS-guided bronchoscopy, while lesion size had no effect. As expected, the position of the probe in relation to the PPLs was found to affect the diagnostic yield of all sampling techniques.
Full table
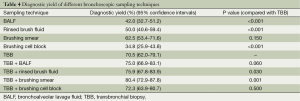
The performance of TBB rendered the highest yield among these specimens (P<0.001, Cochran’s Q test). TBB provided high diagnostic yield irrespective of the size and etiology of the PPLs. Although brushing smear achieved lower diagnostic yield compared with TBB, this difference was not statistically significant (Table 4). The diagnostic yield of brushing smear for benign lesions was quite low, but the size of the PPLs had no influence on the diagnostic performance of this sampling technique. The combination of TBB and brushing smear achieved the maximum diagnostic yield (Table 4).
Full table
Of the 36 benign lesions, 31 lesions were caused by infectious diseases. R-EBUS sampling techniques could give the diagnosis in 23 cases (74.2%), while BALF culture provided microbiological information in 20 cases (64.5%). BALF culture provided additional diagnostic yield for R-EBUS sampling techniques in three cases.
There was no major complication in our study during or after the procedure, and no case of pneumothorax. Minimal self-limiting bleeding was occasionally observed. Only one subject developed pneumonia three days later and responded well to antibiotics.
Discussion
Although several sampling techniques can be used, in many recent reports on the diagnostic yield of R-EBUS-guide FB, only TBB was performed (9-12). At present, there are no recommendations regarding sampling techniques for this bronchoscopic approach. Thus, we conducted this study in order to find out the most suitable sampling technique for this procedure.
TBB provided highest diagnostic yield in our study. Previous reports on the diagnostic yield of this sampling technique for PPLs varied widely, ranging from 46% to 84% (13), and were affected by the lesion size, etiology, CT bronchus sign, and the position of the probe in relation to the PPLs (9,14,15). The recommended number of TBB samplings was at least five biopsy specimens (16).
Since dedicated GS biopsy forceps are smaller than those used in standard biopsies, it is inevitable that the specimens would be smaller. In our early experience, it was difficult for the pathologist to process these samplings as usual standard TBB. Therefore, we adopted the cell block method for this procedure, as previously mentioned. In terms of diagnostic yield, the advantage of the cell block method compared with conventional histological preparation will be a question for future research.
The diagnostic performance of brushing smear cytology in R-EBUS-guided FB has rarely been reported. In two previous studies, brushing smear obtained lower diagnostic yield compared with TBB (14,17). Both TBB and brushing smear sampling techniques have pros and cons. As is the nature of diagnostic methods, histology can reveal not only the morphology of cells, but also additional information such as cell architecture and nearby structure. Moreover, histology sections allow for further investigation of immunohistochemistry and detection of tumor genetic mutations. However, TBB requires that a greater amount of tissue be examined. Although less tissue is obtained via brushing smear, all were smeared in one microscopy slide, which is a sufficient amount for adequate interpretation. Also, a brush can sample the tissue in 360-degree manner with an R-EBUS probe, while TBB can obtain only from a forward angle. Besides, we occasionally observed that for some PPLs, TBB forceps could not be opened inside the lesion initially, but only succeed in getting at the specimen after the brush had been moved back and forth inside the lesion.
We expected that we could obtain the histology using brush technique. However, after investigating the diagnostic yield of cell blocks obtained from this sampling method, we failed to find the benefit. Cell blocks processed from cellular material rinsed from the sampling instrument showed lower diagnostic yield due to the dilution of cellular material (18). Even when the diluent is reduced and the specimens from the brush are gathered in a micropipette tube, the amount may be insufficient for histology processing.
In our study, rinsed brush fluid that was collected from the remainder of the bronchial brushing smear and the material left in the GS obtained low diagnostic yield. Moreover, there was no additional advantage of rinsed brush fluid over brushing smear. Thus, this sampling process should be discarded due to its low cost-effectiveness.
BAL is a sampling technique that does not require any guidance. Compared with our previous study that was performed without guidance (1), even though the mean diameter of PPLs in the present study was smaller, the diagnostic yield was higher (42.0% vs. 29.6%). This might be due to the sequence of the sampling techniques. In the previous study, the routine sequence of bronchoscopic procedure utilized was BAL, brushing, and forceps biopsy. In contrast, in the present study, BAL was performed last, after the lesion had been manipulated by the other sampling instruments, so more cytologic diagnostic cells were detached and could be collected.
There are few data regarding to the diagnostic yield of combine diagnostic sampling modalities. Tay et al. (14) and Kurimoto et al. (17) demonstrated superior yield when brushing and TBB were combined, as in our study. Although the combination of brushing and BAL achieved the maximum diagnostic yield in the study of Kuo et al., TBB plus brushing was not significantly inferior (19).
Although BALF had a lower cytologic diagnostic yield than TBB and brushing smear and did not provide additional benefits for this combination, BALF culture could give additional microbiological information that the other sampling techniques could not. Thus, we suggest that BAL should be a routine procedure after TBB and brushing are performed.
There were some limitations in our study, so that the results may not be directly comparable with previous findings. First, we enrolled only patients who had demonstrated feeding bronchi, because we realized that there had been a diagnostic yield as low as 12% in case of having a negative CT bronchus sign (15). Therefore, in most cases we were able to reach the target confirmed by the EBUS image, which caused the diagnostic yield in our study to be higher compared with the others. Second, the number of PPLs that the probe could reach within the lesion was quite high, compared with previous reports. In fact, we could not place the probe within the lesion on the first attempt in all cases. When an adjacent or negative EBUS image was detected, we moved the bronchoscope with R-EBUS upward and downward and also rotated it in order to find the direction that brought it closer to the target. The R-EBUS probe was then removed slightly and reinserted in that direction. Third, most of PPLs in our study appeared on CT scans as solid PPLs. Limited data regarding the diagnostic yield on ground-glass PPLs indicated unfavorable results, i.e., only 18-65% (9,20). Therefore, our findings are not applicable to all PPLs. Next, we did not randomize the sequence of sampling techniques which might influence to the diagnostic yield. After repeated samplings, the GS sometimes migrates from the proper position due to deep breath or cough, resulting in lower diagnostic yield in late samplings. Therefore, first sampling bias was an issue to debate in our study. Finally, although histology from TBB provided highest the diagnostic yield, we did not perform further processing for immunohistochemical stain and tumor genetic mutations analysis in all cases. Therefore, we did not have data in efficiency and usefulness of TBB on this issue.
In conclusion, we demonstrated that TBB rendered the highest diagnostic yield in R-EBUS-guide FB. The combination of TBB and brushing smear achieved the maximum diagnostic yield. In addition, BAL can provide microbiological data for the appropriate selection of antibiotics in infectious diseases that can present as PPLs. Therefore, to achieve the highest diagnostic performance, TBB, brushing smear and BAL should be performed together.
Acknowledgements
Ethic consideration: This study protocol was approved by the Ethics Committee on Human Experimentation of Ramathibodi Hospital, Faculty of Medicine, Mahidol University.
Authors’ contributions: V Boonsarngsuk: designed and conducted study, performed R-EBUS guided bronchoscopy, analyzed data, prepared and reviewed manuscript. W Kanoksil: processed and reviewed cytology and histology. S Laungdamerongchai: conducted study, assisted R-EBUS guided bronchoscopy.
Disclosure: We have obtained all necessary permissions to publish any figures or tables in the manuscript. The authors declare no conflict of interest.
References
- Boonsarngsuk V, Raweelert P, Sukprapruet A, et al. Factors affecting the diagnostic yield of flexible bronchoscopy without guidance in pulmonary nodules or masses. Singapore Med J 2010;51:660-5. [PubMed]
- Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e142S-65S.
- Baaklini WA, Reinoso MA, Gorin AB, et al. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest 2000;117:1049-54. [PubMed]
- Gasparini S, Ferretti M, Secchi EB, et al. Integration of transbronchial and percutaneous approach in the diagnosis of peripheral pulmonary nodules or masses. Experience with 1,027 consecutive cases. Chest 1995;108:131-7. [PubMed]
- Boonsarngsuk V, Raweelert P, Juthakarn S. Endobronchial ultrasound plus fluoroscopy versus fluoroscopy-guided bronchoscopy: a comparison of diagnostic yields in peripheral pulmonary lesions. Lung 2012;190:233-7. [PubMed]
- Sánchez-Font A, Giralt L, Vollmer I, et al. Endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions. A controlled study with fluoroscopy. Arch Bronconeumol 2014;50:166-71. [PubMed]
- Kurimoto N, Murayama M, Yoshioka S, et al. Analysis of the internal structure of peripheral pulmonary lesions using endobronchial ultrasonography. Chest 2002;122:1887-94. [PubMed]
- Chao TY, Lie CH, Chung YH, et al. Differentiating peripheral pulmonary lesions based on images of endobronchial ultrasonography. Chest 2006;130:1191-7. [PubMed]
- Huang CT, Ho CC, Tsai YJ, et al. Factors influencing visibility and diagnostic yield of transbronchial biopsy using endobronchial ultrasound in peripheral pulmonary lesions. Respirology 2009;14:859-64. [PubMed]
- Fuso L, Varone F, Magnini D, et al. Role of ultrasound-guided transbronchial biopsy in the diagnosis of peripheral pulmonary lesions. Lung Cancer 2013;81:60-4. [PubMed]
- Herth FJ, Ernst A, Becker HD. Endobronchial ultrasound-guided transbronchial lung biopsy in solitary pulmonary nodules and peripheral lesions. Eur Respir J 2002;20:972-4. [PubMed]
- Hsia DW, Jensen KW, Curran-Everett D, et al. Diagnosis of lung nodules with peripheral/radial endobronchial ultrasound-guided transbronchial biopsy. J Bronchology Interv Pulmonol 2012;19:5-11. [PubMed]
- Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest 2012;142:385-93. [PubMed]
- Tay JH, Irving L, Antippa P, et al. Radial probe endobronchial ultrasound: factors influencing visualization yield of peripheral pulmonary lesions. Respirology 2013;18:185-90. [PubMed]
- Evison M, Crosbie PA, Morris J, et al. Can computed tomography characteristics predict outcomes in patients undergoing radial endobronchial ultrasound-guided biopsy of peripheral lung lesions? J Thorac Oncol 2014;9:1393-7. [PubMed]
- Yamada N, Yamazaki K, Kurimoto N, et al. Factors related to diagnostic yield of transbronchial biopsy using endobronchial ultrasonography with a guide sheath in small peripheral pulmonary lesions. Chest 2007;132:603-8. [PubMed]
- Kurimoto N, Miyazawa T, Okimasa S, et al. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest 2004;126:959-65. [PubMed]
- Yung RC, Otell S, Illei P, et al. Improvement of cellularity on cell block preparations using the so-called tissue coagulum clot method during endobronchial ultrasound-guided transbronchial fine-needle aspiration. Cancer Cytopathol 2012;120:185-95. [PubMed]
- Kuo CH, Lin SM, Lee KY, et al. Endobronchial ultrasound-guided transbronchial biopsy and brushing: a comparative evaluation for the diagnosis of peripheral pulmonary lesions. Eur J Cardiothorac Surg 2014;45:894-8. [PubMed]
- Izumo T, Sasada S, Chavez C, et al. The diagnostic utility of endobronchial ultrasonography with a guide sheath and tomosynthesis images for ground glass opacity pulmonary lesions. J Thorac Dis 2013;5:745-50. [PubMed]


