Antimicrobial peptide LL-37 circulating levels in chronic obstructive pulmonary disease patients with high risk of frequent exacerbations
Introduction
Chronic obstructive pulmonary disease (COPD), the fourth leading cause of death in the world, represents an important public health challenge that is both preventable and treatable (1). Exacerbations of COPD increase the decline in lung function, deterioration in health status and risk of death (2-6). The new Global Initiative for Chronic Obstructive Lung Disease strategy (GOLD strategy 2013) included the number of exacerbations, especially severe exacerbations requiring hospital admission, in the grading of COPD (1). The most common cause of COPD exacerbation appears to be respiratory tract infection (viral or bacterial). Host defense peptides are ancient weapons of the innate immunity. In mammals, antimicrobial peptides of the defensin and the cathelicidin families are found. These peptides are expressed on epithelial surfaces and in neutrophils and have been proposed to provide a first line of defence against infection. The only human cathelicidin, human cationic antimicrobial protein 18 (hCAP18), and its C-terminal 37 amino acid fragment (LL-37) have multiple functions, including microbial killing, neutralizing lipopolysaccharide, stimulating leukocyte chemotaxis, promoting angiogenesis and wound healing (7-9). Some studies found that airway concentration of LL-37 (sputum, bronchoalveolar lavage fluid) is up regulated in COPD patients (10,11). However, the relationship between circulating levels of LL-37 and exacerbation risk in COPD patients have not received much attention. The 25-hydroxy vitamin D [25(OH)D] deficiency is associated with COPD and increased susceptibility to infection in the general population (12). In addition, the human cathelicidin antimicrobial peptide gene expression is regulated by the bioactive form of vitamin D (13,14). The objective of our study was to explore the relationship between LL-37 plasma levels, vitamin D status and exacerbation risk in COPD patients.
Methods
Subjects
COPD patients and healthy subjects were recruited from Beijing Hospital, Peking University, China. COPD patients were diagnosed according to the criteria of GOLD strategy (1). The presence of a post-bronchodilator forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) <0.70 confirms the presence of persistent airflow limitation. All patients were in stable clinical condition without reported exacerbation within three months. An exacerbation of COPD is defined as an acute event characterized by a worsening of the patient’s respiratory symptoms that is beyond normal day-to-day variations and leads to a change in medication (1). COPD patients were divided into low risk group (FEV1%pred ≥50% and <2 treated exacerbation per year was considered as low risk) and high risk group (FEV1%pred <50% or ≥2 treated exacerbations per year or ≥1 hospitalizations for COPD exacerbations per year were considered as high risk), depending on the criteria of GOLD strategy (1). Patients who had conditions known to affect plasma concentrations of LL-37 and 25(OH)D, such as cancer, collagen vascular disease, other infection disease, other respiratory disease except COPD were strictly excluded. Healthy subjects had normal physical examinations and showed no symptoms or signs of infection at the time of study. Spirometric test was evaluated in all participants. Data regarding the number of exacerbations in the previous year, smoking history and respiratory symptoms (Modified British Medical Research Council questionnaire, mMRC questionnaire) of COPD patients were evaluated. All participants gave written informed consent with protocols approved by the Institutional Review Boards of Beijing Hospital.
Pulmonary function tests
Pulmonary function tests were performed according to American Thoracic Society guidelines for performance (15). FEV1 and FVC were measured before and after inhaled bronchodilator with standard spirometric techniques (Vmax62 Sensor Medics, Calif, USA). The highest value from at least three spirometric maneuvers was used.
Measurement of LL-37 and vitamin D
Peripheral venous blood samples were taken from all participants. Plasma was separated from blood cells by centrifugation at 1,500 g for 15 min. All samples were stored at −80 °C for subsequent analyzed. The plasma concentrations of LL-37 were measured using ELISA technique (Hycult Biotech, Uden, the Netherlands). The plasma levels of 25(OH)D were measured by electrochemiluminescence immunoassay (ECLIA) on a COBAS e601 ROCHE® analyzer.
Statistical analysis
Data are expressed as mean ± standard deviation (SD) unless otherwise indicated. Statistical comparisons were made using analysis of variance for multiple group comparisons. The relations between variables were evaluated with Pearson’s correlation. A P value <0.05 was considered statistically significant. Data analyses and descriptive statistics were performed with the statistical package for social sciences (SPSS 13.0).
Results
Subject groups
A total of 84 COPD patients and 51 normal subjects (control group) were recruited. COPD patients were divided into low risk group (37 cases) and high risk group (47 cases), depending on FEV1%pred and exacerbation frequency in the previous year. In high risk group, 93.6% patients have had once or more hospitalizations for COPD exacerbations within previous year. There was no significant difference of participants’ age between groups (F=0.334, P=0.717). The post-bronchodilator FEV1%pred and FEV1/FVC were significantly higher in control group than in High Risk group or Low Risk group (P<0.001 for each). The post-bronchodilator FEV1%pred and FEV1/FVC were significantly lower in high risk group than in low risk group (P<0.001). Patient characteristics are shown in Table 1.
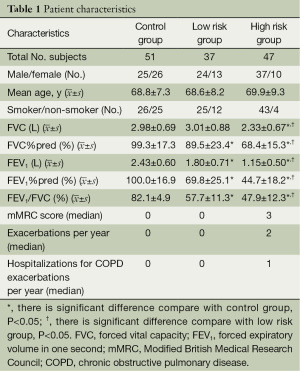
Full table
LL-37 levels in plasma
The plasma concentrations of LL-37 in control group, low risk group and high risk group were 20.7±5.8, 19.5±4.1 and 17.9±3.9 µg/L respectively (Figure 1). The plasma concentration of LL-37 was significantly lower in high risk group than in control group (mean difference −2.75, 95% CI, −4.69 to −0.82, P=0.006). But there was no significant difference between low risk group and high risk group (mean difference 1.53, 95% CI, −0.57 to 3.63, P=0.152). There was no significant difference between low risk group and control group in plasma concentration of LL-37 (mean difference −1.22, 95% CI, −3.27 to 0.82, P=0.239).

25-hydroxy vitamin D [25(OH)D] levels
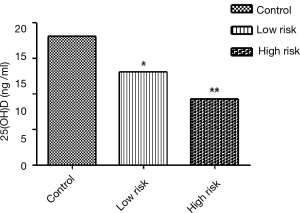
The plasma concentrations of 25(OH)D in control group, low risk group and high risk group were 18.1±9.4, 13.1±6.9 and 9.3±5.8 ng/mL respectively (Figure 2). The plasma concentration of 25(OH)D was significantly higher in control group than in Low Risk group (mean difference 4.98, 95% CI, 1.66-8.30, P=0.004) or high risk group (mean difference 8.75, 95% CI, 5.61-11.89, P<0.001). The plasma concentration of 25(OH)D was significantly lower in high risk group than in low risk group (mean difference −3.78, 95% CI, −7.18 to −0.36, P=0.031).
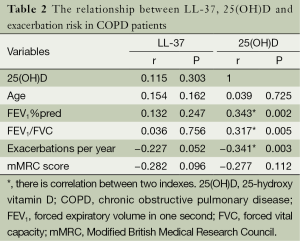
LL-37, 25(OH)D and exacerbation risk
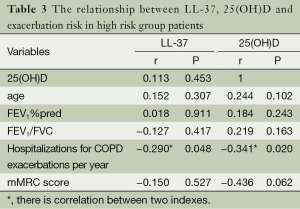
Exacerbation frequency was negative correlated with FEV1%pred (r=−0.395, P=0.001), FEV1/FVC (r=−0.412, P<0.001) and 25(OH)D (r=−0.341, P=0.003) in COPD patients. In high risk group, hospitalization frequency for COPD exacerbations per year was negative correlated with plasma levels of LL-37 (r=−0.290, P=0.048) and 25(OH)D (r=−0.341, P=0.020). There was no significant correlation between LL−37 and 25(OH)D (r=0.115, P=0.303) in COPD patients. The relationship between LL-37, 25(OH)D, age, lung function, syndrome and exacerbation frequency in COPD patients are shown in Tables 2 and 3.
Full table
Full table
Discussion
COPD is a leading cause of morbidity and mortality worldwide and results in an economic and social burden that is both substantial and increasing (16). Exacerbations of COPD are important events in the course of the disease, particularly in those requiring hospitalization. Until now, the best predictor of having frequent exacerbations is a history of previous treated events (requiring treatment with antibiotics and/or systemic corticosteroids) (17). The objective of our study was to seek new predictors of exacerbation risk in COPD patients. Exacerbations of COPD can be triggered by infection with bacteria or viruses, environmental pollutants, or unknown factors. The most common cause appears to be respiratory tract infection (18,19). Antimicrobial peptides play an important role in innate defense against infection (20).
We measured LL-37, the only human cathelicidin antimicrobial peptide, in COPD patients. We found that plasma levels of LL-37 in COPD patients with high risk of frequent exacerbation were lower than normal subjects. And LL-37 levels inversely associated with the frequency of hospitalization for COPD exacerbations in these high risk patients. Most of the previous studies have focused on the local innate immunity role of LL-37, only a few of them have investigated the circulating levels of LL-37 in COPD patients. To our knowledge, the relationship between circulating levels of LL-37 and exacerbation risk has not been previously described in patients with COPD. Xiao et al. (21) found that hCAP18 levels in induced sputum were elevated in COPD patients compared to control subjects and inversely correlated with pulmonary function. Parameswaran et al. (10) reported that sputum LL-37 levels were higher during COPD exacerbation compared with baseline. A small-scale research showed that increased induced sputum levels of LL-37 in COPD patients were associated with airflow limitation, health status and exercise tolerance compared with control group (22). But there were no statistically significant differences in serum LL-37 levels between healthy smokers, healthy non-smokers, COPD patients with FEV1% more than 50% and less than 50% (22). These results suggested that airway levels of LL-37 are likely to be important in pathogen clearance and clinical outcomes of infection in COPD patients. About the difference between circulating and airway levels of LL-37, we presumed that the increase of airway levels of LL-37 is a local immune response to outside stimulation and plasma levels of LL-37 reflects status of systemic innate immunity. The decline of defence function against infection leads to frequent exacerbations of COPD. The decrease of circulating levels of LL-37 might be increase the risk of exacerbation in COPD patients. Previous research about patients admitted to intensive care units partly confirmed our inference, mean plasma LL-37 levels were significantly lower in critically ill subjects compared to healthy controls (23).
A growing body of evidences suggest an important role for vitamin D in mounting appropriate innate and adaptive immune responses to infections (24). In our study, plasma concentration of 25(OH)D was significantly higher in normal subjects than in COPD patients. The plasma concentrations of 25(OH)D in COPD patients with high risk of frequent exacerbation were significantly lower than in low risk COPD patients. Hospitalization frequency for COPD exacerbations was negative correlated with plasma levels of 25(OH)D in COPD patients with high risk of frequent exacerbation. Previous studies also show that Vitamin D insufficiency and deficiency are highly prevalent in patients with COPD, with the lowest vitamin D levels being associated with the most severe airflow obstruction (12). Quint et al. (25) considered that low 25-hydroxyvitamin D levels in COPD were not associated with frequent exacerbations. However, Lehouck et al. (26) found that high-dose vitamin D supplementation may reduce exacerbations in patients with severe vitamin D deficiency at baseline. Some previous studies found the ability of 1,25(OH)2D to increase expression of antimicrobial peptides (13,14). In our study, we did not find significant correlation between plasma levels of LL-37 and 25(OH)D. Human cationic antimicrobial protein 18 gene expression, LL-37 peptide release and transformation from 25(OH)D to 1,25(OH)2D were impacted by many factors in vivo environment. The regulation of LL-37 levels is a complex process in COPD patients, vitamin D is not the only influencing factor of circulating levels of LL-37.
Based on our data, the plasma levels of LL-37 and 25(OH)D were lower in COPD patients with high risk of frequent exacerbations than normal subjects. Hospitalization frequency for COPD exacerbations was negative correlated with plasma levels of LL-37 and 25(OH)D in high risk COPD patients. So we speculate that low plasma levels of LL-37 and 25(OH)D might be predictors of exacerbation risk in COPD patients, but further large-scale work is necessary to confirm this inference.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Fabbri L, Pauwels RA, Hurd SS, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: GOLD Executive Summary updated 2003. COPD 2004;1:105-41; discussion 103-4. [PubMed]
- Bourbeau J, Ford G, Zackon H, et al. Impact on patients' health status following early identification of a COPD exacerbation. Eur Respir J 2007;30:907-13. [PubMed]
- Kessler R, Ståhl E, Vogelmeier C, et al. Patient understanding, detection, and experience of COPD exacerbations: an observational, interview-based study. Chest 2006;130:133-42. [PubMed]
- Donaldson GC, Seemungal TA, Bhowmik A, et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002;57:847-52. [PubMed]
- Kanner RE, Anthonisen NR, Connett JE, et al. Lower respiratory illnesses promote FEV(1) decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health study. Am J Respir Crit Care Med 2001;164:358-64. [PubMed]
- Spencer S, Calverley PM, Burge PS, et al. Impact of preventing exacerbations on deterioration of health status in COPD. Eur Respir J 2004;23:698-702. [PubMed]
- Sørensen O, Arnljots K, Cowland JB, et al. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood 1997;90:2796-803. [PubMed]
- Bals R, Wang X, Zasloff M, et al. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci U S A 1998;95:9541-6. [PubMed]
- Nizet V, Ohtake T, Lauth X, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 2001;414:454-7. [PubMed]
- Parameswaran GI, Sethi S, Murphy TF. Effects of bacterial infection on airway antimicrobial peptides and proteins in COPD. Chest 2011;140:611-7. [PubMed]
- Golec M, Reichel C, Lemieszek M, et al. Cathelicidin LL-37 in bronchoalveolar lavage and epithelial lining fluids from COPD patients and healthy individuals. J Biol Regul Homeost Agents 2012;26:617-25. [PubMed]
- Janssens W, Bouillon R, Claes B, et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax 2010;65:215-20. [PubMed]
- Wang TT, Nestel FP, Bourdeau V, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol 2004;173:2909-12. [PubMed]
- Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006;311:1770-3. [PubMed]
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. [PubMed]
- Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J 2006;27:397-412. [PubMed]
- Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010;363:1128-38. [PubMed]
- Rosell A, Monsó E, Soler N, et al. Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Arch Intern Med 2005;165:891-7. [PubMed]
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 2008;359:2355-65. [PubMed]
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature 2002;415:389-95. [PubMed]
- Xiao W, Hsu YP, Ishizaka A, et al. Sputum cathelicidin, urokinase plasminogen activation system components, and cytokines discriminate cystic fibrosis, COPD, and asthma inflammation. Chest 2005;128:2316-26. [PubMed]
- Jiang YY, Xiao W, Zhu MX, et al. The effect of human antibacterial peptide LL-37 in the pathogenesis of chronic obstructive pulmonary disease. Respir Med 2012;106:1680-9. [PubMed]
- Jeng L, Yamshchikov AV, Judd SE, et al. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J Transl Med 2009;7:28. [PubMed]
- Ginde AA, Mansbach JM, Camargo CA Jr. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med 2009;169:384-90. [PubMed]
- Quint JK, Donaldson GC, Wassef N, et al. 25-hydroxyvitamin D deficiency, exacerbation frequency and human rhinovirus exacerbations in chronic obstructive pulmonary disease. BMC Pulm Med 2012;12:28. [PubMed]
- Lehouck A, Mathieu C, Carremans C, et al. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med 2012;156:105-14. [PubMed]


