
Prognosis of wide wedge resection in patients with stage IA1 and IA2 lung adenocarcinoma with total tumor size including the lepidic component greater than 2 cm: a single center retrospective study
Introduction
There was a significant change in the staging of lung adenocarcinoma in the eighth edition of the TNM (T for primary tumor, N for nodal involvement, M for metastasis) staging system (1). In particular, the tumor size in the seventh edition was measured by total tumor size plus the lepidic component. In the eighth edition of the TNM staging system, this has been changed to include the invasive component of the tumor (2). Therefore, tumors found to be larger than 2 cm based on the seventh edition of the TNM staging system may actually be reclassified as smaller than 2 cm based on the eighth edition. Because the TNM stage is the most important predictor of prognosis for lung cancer, changes in the T stage may also mean changes in treatment options (3).
Recently, sublobar resection was considered to be adequate treatment for small-sized lung adenocarcinoma (4-7). Especially, lepidic predominant adenocarcinomas, such as adenocarcinoma in situ, minimally invasive adenocarcinoma, and lepidic adenocarcinoma, have a good prognosis after sublobar resection (4,8). However, most of the studies performed sublobar resection when the tumor size was less than 2 cm, and the seventh edition of the TNM staging system was used to determine the tumor size, which included the lepidic component (9,10). In the eighth edition of the TNM staging system, however, the size of the lepidic component is excluded and the tumor size is measured only by the size of the invasive component. Therefore, when evaluating indications for sublobar resection, it is necessary to determine whether the size of the invasive component is less than 2 cm, rather than the total tumor size including the lepidic component. Even if the size of the invasive component is less than 2 cm, total tumor sizes (including the lepidic component) larger than 2 cm do exist, so it is necessary to determine whether sublobar resection is appropriate in these cases.
For small peripheral lung adenocarcinomas, wedge resection can be easily performed, and there are studies that have demonstrated a good prognosis after surgery (5,11-13). We reported a good prognosis after wedge resection for non-small cell lung cancer (NSCLC) with an invasive component of less than 2 cm (14). However, even if the invasive component is less than 2 cm, it is not known whether the wedge resection will result in a good prognosis even when the total tumor size, including the lepidic component, is larger than 2 cm.
The purpose of this study is to evaluate the prognosis of wide wedge resection in patients with stage IA1 and IA2 lung adenocarcinoma in which the total tumor size is greater than 2 cm. Therefore, this study seeks to determine whether the size of the lepidic component in lung adenocarcinoma can be ignored and sublobar resection can be performed based solely on the size of the invasive component. We present the following article in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-1507).
Methods
Patients
From 2010 to 2018, 1,994 consecutive patients at a hospital in Korea were diagnosed with NSCLC and underwent curative surgery. Among those patients, 855 patients were diagnosed with stage IA1 and IA2 lung adenocarcinoma according to the eighth edition of the TNM staging system. Patients with incomplete resection or those who had preoperative chemotherapy were excluded. In this study, only stage IA1 and IA2 lung adenocarcinoma with total tumor size including lepidic component more than 2 cm were included. Total tumor sizes of all study patients were greater than 2 cm, but all of them were in stage IA1 and IA2 because the invasive component sizes were within 2 cm according to the eighth edition of the TNM staging system. The surgical procedures were either wide wedge resection or anatomical lobectomy. To reduce selection bias, all data were obtained from consecutive patient data. Finally, 180 consecutive patients were reviewed retrospectively. The patients were determined as stage IA1 or stage IA2, and their clinicopathological characteristics and prognosis were compared between the wedge resection group and the lobectomy group in patients with stage IA1 and stage IA2, respectively. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the institutional review board of Seoul St. Mary’s Hospital at the Catholic University of Korea and individual consent was waived (referral No.: KC20RISI0210).
Surgical procedures
The standard surgical procedure for stage IA lung cancer was anatomical lobectomy and systematic nodal dissection. However, in the case of peripheral ground glass opacity (GGO) tumors, sublobar resection, such as wedge resection or anatomical segmentectomy, was also considered. Although the tumor size was greater than 2 cm, sublobar resection was considered if the size of the invasive component was less than 2 cm. In this study, only wedge resection cases were included. Wedge resection was defined as non-anatomical parenchymal resection of the lung irrespective of vessel or bronchus division. Wide wedge resections were performed to get sufficient resection margin distance. In this study, we defined wide wedge resection as wedge resection with a sufficient resection margin of more than 2 cm. In all cases, a sufficient resection margin was obtained, in which the margin length was greater than the total tumor size. In the case of patients considered high risk (decreased pulmonary function or comorbid disease), wedge resection was usually performed. Wedge resection procedures were achieved using endostaplers.
Eighth edition of TNM staging system and histological evaluation
Patients diagnosed with clinical stage I lung cancer on chest computed tomography (CT) and positron emission tomography/CT scan were eligible for surgical treatment. Tumor specimens were measured again by the pathologist to reclassify the pathologic T stage according to the eighth edition of the TNM classification (3), and the T stage was defined by the greatest dimension of the invasive component of the tumor (2). Total tumor size was defined as the maximal diameter of tumor including the lepidic component. Pathology reports were reviewed for tumor size, location, histologic tumor grade, lymph node status, and lymphovascular invasion. Lymphovascular invasion was defined as tumor cells microscopically observed in the lymphatic or vascular lumen.
Statistical analysis
Patients were grouped according to tumor stage and surgical procedure. Clinicopathological factors were compared using a Student’s t-test or the Wilcoxon rank sum test for continuous variables and chi-squared or Fisher exact test for categorical variables. The Kaplan-Meier method was used to analyze data collected from the interval between the time of operation and the time of the last follow-up visit. Recurrence-free survival (RFS) rates were estimated by the Kaplan-Meier method. Survival of each group was compared by log-rank test. A Cox proportional hazards model was used in a multivariate analysis to identify risk factors for recurrence after surgery. All variables with P<0.10 on univariate analysis were entered into the multivariate analysis. A P value of less than .05 was considered statistically significant. Statistical analysis was performed using SPSS version 24.0 software (IBM Corp, Armonk, NY, USA).
Results
Wedge resection group versus lobectomy group in stage IA1
Stage IA1 cancer was diagnosed in 54 patients. Fourteen patients underwent wedge resection and 40 patients underwent anatomical lobectomy. Table 1 shows the comparison of clinicopathological characteristics between the wedge resection group and the lobectomy group. The clinicopathological variables were not statistically different between the two groups. In the surgical outcomes, the duration of postoperative chest drainage and the duration of hospital stay were shorter in the wedge resection group than in the lobectomy group. There was no postoperative mortality.
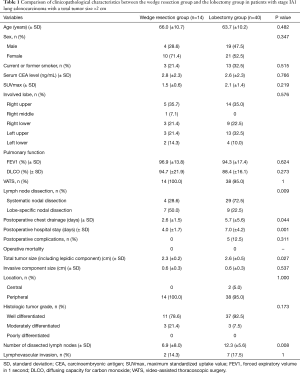
Full table
Table 2 shows the reasons for wedge resection in patients with stage IA1 lung adenocarcinoma. Ten patients (71.4%) underwent wedge resection intentionally. Others underwent wedge resection due to underlying lung disease (2, 14.3%) or previous history of contralateral lung operation (2, 14.3%).
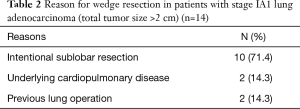
Full table
The median follow-up period for the wedge resection group and lobectomy group was 1,315 days (range, 405–2,155 days) and 1,448 days (range, 81–3,290 days), respectively. The 5-year RFS rates were 100% in both groups. There were no recurrences and cancer-related deaths during the follow-up period. Only two patients died due to liver disease (at 81 days and 3,187 days) in the lobectomy group.
Wedge resection group versus lobectomy group in stage IA2
Stage IA2 was diagnosed in 126 patients. Sixteen patients underwent wedge resection and 110 patients underwent anatomical lobectomy. Table 3 shows the comparison of clinicopathological characteristics between the wedge resection group and the lobectomy group. There were no statistical differences in clinicopathological characteristics between the two groups. The duration of postoperative chest drainage was shorter in the wedge resection group than that in the lobectomy group.
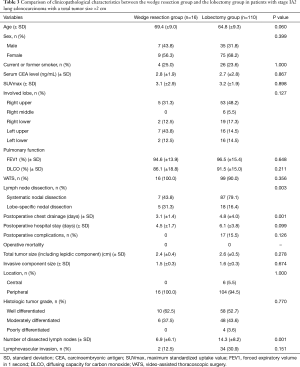
Full table
Table 4 shows the reasons for wedge resection in patients with stage IA2 lung adenocarcinoma. Eleven patients (68.8%) underwent wedge resection intentionally. One patient (6.3%) underwent wedge resection because of underlying pulmonary disease. Four patients (25.0%) underwent wedge resection due to a previous history of contralateral lung operation.

Full table
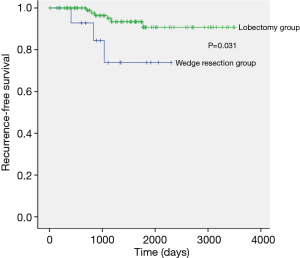
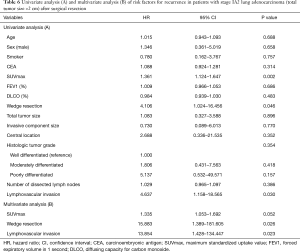
The median follow-up period of the wedge resection group and the lobectomy group was 1,041 days (range, 146–2,393 days) and 1,469 days (range, 173–3,502 days), respectively. There were 7 recurrences in stage IA2 lung adenocarcinoma and the distribution of the recurrence sites was not different between the wedge resection group and the lobectomy group (Table 5). The 5-year RFS rates for the wedge resection group and the lobectomy group were statistically significant (73.9% and 90.8%, respectively; P=0.031) (Figure 1). Univariate and multivariate analysis of risk factors for recurrence in patients with stage IA2 lung adenocarcinoma after surgical resection were conducted by using Cox proportional hazard model (Table 6). The variables with P values less than 0.10 were maximum standardized uptake value, wedge resection, and lymphovascular invasion. Those variables with P values less than 0.10 were entered into the multivariate analysis. In the multivariate analysis, wedge resection [hazard ratio (HR) =15.883; P=0.026] and lymphovascular invasion (HR =13.854; P=0.023) were significant risk factors for recurrence in patients with stage IA2 lung adenocarcinoma after surgical resection.

Full table
Full table
Discussion
For stage I NSCLC, anatomical lobectomy has been recommended regardless of tumor size (15). Although anatomical lobectomy remains the standard treatment for early stage lung cancer, there are many studies that have reported that sublobar resection has a good prognosis for the treatment of small-sized (≤2 cm) NSCLC (4,6,7,14,16-19). Sublobar resection is regularly performed in lung adenocarcinoma presenting as a GGO nodule on chest CT. GGO is strongly correlated with a lepidic component of adenocarcinoma. In fact, adenocarcinoma including a lepidic component is regarded as a low-grade malignancy (20-22). Some studies have reported that adenocarcinoma with a lepidic component has a better prognosis than other NSCLCs of the same stage (23-25). Thus, sublobar resection is generally performed for adenocarcinoma containing a lepidic component. However, most studies on sublobar resection have been performed only when the maximal diameter of the tumor is less than 2 cm (9,10). Since the lepidic component of adenocarcinoma is considered malignant without invasiveness (26), the eighth edition of the TNM staging system performs T staging based only on the size of the invasive component but not the lepidic component. Therefore, the tumor size as an indication of sublobar resection should be studied by applying a new staging system. If the eighth edition of the staging system is applied, lepidic adenocarcinoma greater than 2 cm can be diagnosed as T1a with an invasive component less than 1 cm or T1b with an invasive component less than 2 cm. In this study, 180 patients with T1a (stage IA1) and T1b (stage IA2) tumors whose total tumor size exceeded 2 cm (invasive component ≤2 cm) were studied. Although the total tumor size was more than 2 cm, the 5-year RFS rate was 100% for patients with stage IA1 tumors after wedge resection. On the other hand, the recurrence rate after wedge resection was higher than that after lobectomy in stage IA2 tumors. Moreover, wedge resection was a significant poor prognostic factor in multivariate analysis. Therefore, when the eighth edition of TNM staging system is applied to tumors larger than 2 cm in size, patients with stage IA1 lung adenocarcinoma can expect a good prognosis after wide wedge resection. However, for patients with stage IA2 lung adenocarcinoma, lobectomy has better results than wide wedge resection does.
For a lung nodule located near the visceral pleura, it is very easy to perform wedge resection. Some studies reported that lung adenocarcinoma presenting as GGO on chest CT has a good prognosis after wedge resection (7,12,27). Wedge resection is much simpler to perform than segmentectomy or lobectomy, as it needs short surgery time but results in fast postoperative recovery. In this study, the duration of chest drainage or hospital stay was shorter in the wedge resection group than that in the lobectomy group. Thus, in the case of lung adenocarcinoma located very close to the visceral pleura, applying wide wedge resection can reduce the surgeon and patient burden. Of course, all cases of wedge resection achieved sufficient resection margin. Although all tumors were located in the periphery, a relatively wide lung parenchyma was resected to ensure sufficient resection margin distance, which we call a wide wedge resection.
In this study, the sample size of the wedge resection groups was very small. Therefore, it is difficult to generalize the results. In the seventh edition of the TNM staging system, wedge resection was not generally recommended when total tumor size was greater than or equal to 2 cm because it was classified as T1b or higher. However, in the eighth edition, even if the total tumor size is larger than 2 cm, it is sometimes classified as T1a because of a small invasive component size. Therefore, it is necessary to re-evaluate the prognosis of wedge resection. This study was a retrospective study, and in the past, if the total tumor size was greater than 2 cm, wedge resection was rarely performed. As a result, sufficient samples could not be collected. Nevertheless, the results of this study are not meaningless because the sample size is such that wedge resection and lobectomy can be compared statistically. The purpose of this study is to become a baseline study of large-scale studies to be conducted in the future.
We did not include segmentectomy in this study because of a relatively small number of patients. In fact, segmentectomy is a more complex procedure than wedge resection (5). In some cases, segmentectomy is considered a more difficult surgical procedure to perform than lobectomy. Wedge resection is always easier than any anatomical resection (e.g., segmentectomy or lobectomy), which is its most important advantage over other surgical procedures. In some cases of wide wedge resection, the lung volume of the resected lung parenchyma was similar to that of segmentectomy. Therefore, the resection margin distance of wide wedge resection was not significantly different from that of segmentectomy. In our institution, wide wedge resection tended to be preferred over segmentectomy for peripheral GGO. Thus, the cases in which wide wedge resection were performed were collected and analyzed in this study.
In our previous research, we compared the prognosis of wedge resection and lobectomy in stage IA1 and IA2 NSCLC according to the eighth edition of the TNM staging system (14). We found that there was no statistical difference in RFS for wedge resection and lobectomy at stages IA1 and IA2 NSCLC in the eighth edition. On the other hand, the prognosis of wide wedge resection in stage IA1 adenocarcinoma was adequate in this study, but recurrence rate was significantly higher in stage IA2 adenocarcinoma. The main difference between the previous and current research is that the current study was conducted on lung adenocarcinoma with a total tumor size larger than 2 cm. The current study shows that in stage IA2 lung adenocarcinoma, wide wedge resection may be an inappropriate procedure if the total tumor size including the lepidic component is more than 2 cm.
This study has a few limitations. First, it was a retrospective review. Second, we obtained data from a single institution, and the sample size was relatively small to generalize the results more reliably. However, the use of very detailed data was possible because of the comprehensive information stored in the electronic medical record. We retrieved data that thoroughly described the surgical procedures along with exhaustive data from pathologic specimens and pathologic reports. We believe that our data will be useful as the basis for future investigations. A prospective, randomized controlled study should be performed to validate our results. Third, this study used only the pathological stage, not the clinical stage. Whether to perform wedge resection or lobectomy before surgery is determined by the clinical stage. Therefore, it is true that research using the clinical stage is more helpful for actual clinical use. However, because the pathological stage is more accurate and has less variation, before conducting a study using the clinical stage, studies using the pathological stage must first be performed. This study was also conducted using the pathological stage, and further studies using the clinical stage will be conducted in the future. Finally, the follow-up period of the wedge resection group was relatively short. The median follow-up period of the stage IA2 wedge resection group was 35 months. The median follow-up period of the total patients was 45 months. In the future, it is expected that more accurate research results can be derived if more and longer follow-up data are accumulated.
In conclusion, wide wedge resection for stage IA1 lung adenocarcinoma with a total tumor size larger than 2 cm had a prognosis comparable to that of lobectomy, whereas wide wedge resection had a higher recurrence rate than lobectomy in patients with stage IA2 lung adenocarcinoma with total tumor size larger than 2 cm. Therefore, if the total tumor size including the lepidic component is more than 2 cm, a good prognosis can be expected with wide wedge resection if it is in the stage IA1 category. Further research through multicenter randomized controlled trials may more accurately depict patient outcomes.
Acknowledgments
A native English-speaking professional (BioMed Proofreading, LLC) refined the written content.
Funding: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2019R1G1A1099670).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1507
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1507
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-20-1507
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1507). YM reports grants from National Research Foundation of Korea (NRF), during the conduct of the study. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the institutional review board of Seoul St. Mary’s Hospital at the Catholic University of Korea and individual consent was waived (Referral number: KC20RISI0210).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rami-Porta R, Asamura H, Travis WD, et al. Lung cancer - major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:138-55.
- Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1204-23.
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Zhao ZR, Situ DR, Lau RWH, et al. Comparison of Segmentectomy and Lobectomy in Stage IA Adenocarcinomas. J Thorac Oncol 2017;12:890-6. [Crossref] [PubMed]
- Altorki NK, Kamel MK, Narula N, et al. Anatomical Segmentectomy and Wedge Resections Are Associated with Comparable Outcomes for Patients with Small cT1N0 Non-Small Cell Lung Cancer. J Thorac Oncol 2016;11:1984-92. [Crossref] [PubMed]
- Moon Y, Lee KY, Park JK. Prognosis After Sublobar Resection of Small-sized Non-small Cell Lung Cancer with Visceral Pleural or Lymphovascular Invasion. World J Surg 2017;41:2769-77. [Crossref] [PubMed]
- Moon Y, Lee KY, Park JK. The prognosis of invasive adenocarcinoma presenting as ground-glass opacity on chest computed tomography after sublobar resection. J Thorac Dis 2017;9:3782-92. [Crossref] [PubMed]
- Aokage K, Yoshida J, Hishida T, et al. Limited resection for early-stage non-small cell lung cancer as function-preserving radical surgery: a review. Jpn J Clin Oncol 2017;47:7-11. [Crossref] [PubMed]
- Asamura H, Aokage K, Yotsukura M. Wedge Resection Versus Anatomic Resection: Extent of Surgical Resection for Stage I and II Lung Cancer. Am Soc Clin Oncol Educ Book 2017;37:426-33. [Crossref] [PubMed]
- Suzuki K, Saji H, Aokage K, et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J Thorac Cardiovasc Surg 2019;158:895-907. [Crossref] [PubMed]
- Maurizi G, D'Andrilli A, Ciccone AM, et al. Margin Distance Does Not Influence Recurrence and Survival After Wedge Resection for Lung Cancer. Ann Thorac Surg 2015;100:918-24; discussion 924-5. [Crossref] [PubMed]
- Cho JH, Choi YS, Kim J, et al. Long-term outcomes of wedge resection for pulmonary ground-glass opacity nodules. Ann Thorac Surg 2015;99:218-22. [Crossref] [PubMed]
- Cao J, Yuan P, Wang Y, et al. Survival Rates After Lobectomy, Segmentectomy, and Wedge Resection for Non-Small Cell Lung Cancer. Ann Thorac Surg 2018;105:1483-91. [Crossref] [PubMed]
- Moon Y, Park JK, Lee KY, et al. Prognosis after wedge resection in patients with 8(th) edition TNM stage IA1 and IA2 non-small cell lung cancer. J Thorac Dis 2019;11:2361-72. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Taioli E, Yip R, Olkin I, et al. Survival after Sublobar Resection for Early-Stage Lung Cancer: Methodological Obstacles in Comparing the Efficacy to Lobectomy. J Thorac Oncol 2016;11:400-6. [Crossref] [PubMed]
- Khullar OV, Liu Y, Gillespie T, et al. Survival After Sublobar Resection versus Lobectomy for Clinical Stage IA Lung Cancer: An Analysis from the National Cancer Data Base. J Thorac Oncol 2015;10:1625-33. [Crossref] [PubMed]
- Moon Y, Park JK, Lee KY. The Effect of Resection Margin Distance and Invasive Component Size on Recurrence After Sublobar Resection in Patients With Small (</=2 Cm) Lung Adenocarcinoma. World J Surg 2020;44:990-7. [Crossref] [PubMed]
- Cao C, Chandrakumar D, Gupta S, et al. Could less be more?-A systematic review and meta-analysis of sublobar resections versus lobectomy for non-small cell lung cancer according to patient selection. Lung Cancer 2015;89:121-32. [Crossref] [PubMed]
- Hung JJ, Yeh YC, Wu YC, et al. Prognostic Factors in Completely Resected Node-Negative Lung Adenocarcinoma of 3 cm or Smaller. J Thorac Oncol 2017;12:1824-33. [Crossref] [PubMed]
- Moon Y, Sung SW, Lee KY, et al. The importance of the lepidic component as a prognostic factor in stage I pulmonary adenocarcinoma. World J Surg Oncol 2016;14:37; discussion 636. [Crossref] [PubMed]
- Sasada S, Nakayama H, Miyata Y, et al. Comparison of malignant grade between pure and partially invasive types of early lung adenocarcinoma. Ann Thorac Surg 2015;99:956-60. [Crossref] [PubMed]
- Hattori A, Hirayama S, Matsunaga T, et al. Distinct Clinicopathologic Characteristics and Prognosis Based on the Presence of Ground Glass Opacity Component in Clinical Stage IA Lung Adenocarcinoma. J Thorac Oncol 2019;14:265-75. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Takamochi K, et al. Indications for sublobar resection of clinical stage IA radiologic pure-solid lung adenocarcinoma. J Thorac Cardiovasc Surg 2017;154:1100-8. [Crossref] [PubMed]
- Moon Y, Kim KS, Sung SW, et al. Correlation of histological components with tumor invasion in pulmonary adenocarcinoma. World J Surg Oncol 2014;12:388. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Sim HJ, Choi SH, Chae EJ, et al. Surgical management of pulmonary adenocarcinoma presenting as a pure ground-glass nodule. Eur J Cardiothorac Surg 2014;46:632-6. [Crossref] [PubMed]


