Clinical features and mutation status of EGFR, KRAS, BRAF, EML4-ALK and ROS1 between surgical resection samples and non surgical resection samples in lung cancer
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide, and more than 1.4 million deaths every year (1). Despite improvements in early detection and novel treatment for lung cancer in recent years, the prognosis of lung cancer has remained poor, and the 5-year survival is below 20% in most countries, the 5-year survival is about 18% in China (2). There are two major types of lung cancer, small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), and about 85% of lung cancer is NSCLC, which contains three major histologic subtypes: adenocarcinoma (ADC), squamous cell carcinoma (SCC) and large cell carcinoma (LCC). NSCLC is often diagnosed at an advanced stage (3). For early-stage NSCLC, the standard and potentially efficiency treatment is surgical resection, and for the advanced NSCLC, chemotherapy and target therapy is necessary (4).
With the development of sequencing, the whole genome sequencing and exome sequencing of lung cancer were carried out, and more and more driver mutations were detected in lung cancer, including epidermal growth factor receptor (EGFR), KRAS-oncogene, and anaplastic lymphoma kinase (ALK) (5-7). Fully understanding the molecular variations not only points out novel insight into development of lung cancer, but also provides specific molecular targets for therapeutic intervention. Target therapy is very common in lung cancer therapy and as the first-line treatment for lung cancer. Several such targeted therapies, including EGFR tyrosine kinase inhibitors (erlotinib and gefitinib) and ALK inhibitor (crizotinib), have shown significant clinical efficacy in treating patients with NSCLC harboring corresponding gene mutations (8,9). Hence the detection of somatic mutation of EGFR, KRAS and EML4-ALK fusion is essential for the decision to make an appropriate treatment.
As we all know, lung cancer is a heterogeneous disease, so accurate detection of driver gene mutations is very important for clinical treatment of lung cancer, especially for the advance lung cancer patients who lose the opportunity of surgical resection, because we can only obtain some small biopsy samples. In order to evaluate mutations of EGFR, KRAS, and EML4-ALK gene fusion between surgical tissues and biopsy samples, 1,357 surgical tissues and 145 biopsy samples were collected in this study to carry out the detection.
Materials and methods
Ethical approval
The study was approved by the Institutional Review Board (IRB) of Shanghai Pulmonary Hospital affiliated Tongji University. Written informed consents were obtained from all participants or their guardians.
Sample collection
A total of 1,357 surgical tissues and 145 biopsy samples histopathologically diagnosed with lung cancer from Shanghai pulmonary hospital affiliated to Tongji University from January 2014 to January 2015 were collected in this study. No patients received any anticancer therapies before detection. All samples were evaluated by pathologists and meet the criteria of containing at least 50% tumor cells. Patients with insufficient or poor-quality tissue for molecular analyses were excluded.
Mutation detection
Genomic DNA and total RNA were extracted from fresh tissues using QIAamp DNA Tissue Kit and RNeasy Kit (Qiagen, Germany), respectively. Mutations of EGFR and KRAS were detected by commercially available kits from ACCB Biotech (Beijing, China). The kit is based on the Taqman technology. The EGFR kit can detect 44 mutations in exon 18-21, including G719S/C/A in exon 18, L858R and L861Q in exon 21, T790M and S768I in exon 20, 6 insertions in exon 20, 31 deletions in exon 19. The KRAS kit can detect Gly12Asp, Gly12Ala, Gly12Val, Gly12Ser, Gly12Arg, Gly12Cys, and Gly13Asp in codon 12 and 13. The rearrangement of EML4-ALK were detected using kit from Amoy Diagnostics (Xiamen, China), the kit was based on amplification refractory mutation system (ARMS) real-time PCR technology. This kit can detect 7 fusions (variant 1, 2, 3a/b, 5a/b, and 5’). All tests were performed according to the manufacturer’s protocol.
Clinical information collection
In order to investigate the difference between surgical tissues and biopsy tissues, the clinical information, such as age, gender, histological lung cancer subtypes, and stage. The stage of lung cancer was confirmed according to the Union International Contre le Cancer (UICC-7) staging system for lung cancer (10).
Statistical analysis
Alterations between different subgroups stratified by gender and age status were analyzed by Chi-square and Fisher’s exact tests when appropriate. Pearson χ2 test (when no cell of a contingency table has expected count less than five) or Fisher’s exact tests (when any cell of a contingency table has expected count less than five) was used to assess the association between two categorical variables with SPSS for Windows (Version 17.0, Chicago, IL, USA). All P values presented were two-sided, and a level of P<0.05 was considered statistically significant.
Results
Sample characteristics
In order to analysis the association of distribution of driver gene mutations between SRSs and NSRSs in lung cancer, 1,357 SRSs and 145 NSRSs histopathologically diagnosed with lung cancer from Shanghai pulmonary hospital affiliated to Tongji University from January 2014 to January 2015 were collected in this study. The 145 biopsy samples contained 81 puncture samples, 10 hydrothorax samples, 37 liquid based cytologic test (LCT) samples, 11 endobroncheal ultrasonography (EBUS) samples, and magnetic navigation samples.
We collected the clinical information, such as age, gender, grade and histological type and so on, to analysis in this study. The mean age was 60.50±9.84 in surgical resection patients, and 60.50±10.28 in non surgical resection patients, there was no significant difference in age between two groups (P=0.998). The results also showed that there were no significant differences between SRSs and NSRSs in gender (P=0.601), and histological type (P=0.185), the main histological type of lung cancer was ADE. The significant result was found in grade (P<0.001), the majority cases in SRSs were in gradeI, however, more grade IV cases were found in NSRSs (Table 1).
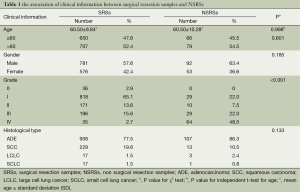
Full table
Difference of age and gender in different histological type of lung cancer between SRSs and NSRSs
In order to analysis the difference of age and gender in different histological type of lung cancer between SRSs and NSRSs, independent t-test was used to compare the mean age in ADE, SCC, LCLC, and SCLC, and χ2 test was performed to evaluate the difference of sex in different groups. The results were shown in Table 2. The results showed that there was no significant difference in age between SRSs and NSRSs (P>0.05). However, the significant result was found between ADE and other groups (P<0.001), the ADE patients presented younger than other groups. There were no differences between other groups. In gender analysis, more females in SRSs, but more males in NSRSs group (P=0.024). There were no significant differences in other types.
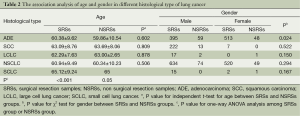
Full table
There were no significant differences of EGFR mutations between SRSs and NSRSs groups
To assess the difference of EGFR mutations between SRSs and NSRSs, we detected 44 mutations of EGFR in exon 18-21. The results showed in Table 3. There were no significant differences of EGFR mutations between SRSs and NSRSs groups expect di-mutation which presented two mutations in the one patient at the same time. The L858R in exon 21 is the most common mutation in lung cancer, and exon 19 deletion (19-del) is the second one, and they two accounted for over 90% mutations of EGFR.
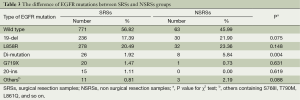
Full table
There were no significant differences of KRAS, BRAF, EML4-ALK and ROS1 mutations between SRSs and NSRSs groups
We also detected the mutations of KRAS, BRAF, EML4-ALK and ROS1 in different groups, because the mutation rate is too low, we did not presented certain type of mutation. Table 4 showed the results. The mutation rate is 8.39% and 7.04% in SRSs and NSRSs, respectively. The mutation of BRAF only detected in SRSs, and mutation rate is 0.21%. The mutation rate of EML4-ALK and ROS1 in both groups was about 5%. There were no significant differences of KRAS, BRAF, EML4-ALK and ROS1 mutations between SRSs and NSRSs groups.
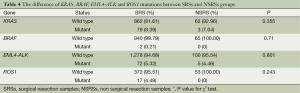
Full table
Discussion
In this study, we collected 1,357 SRSs and 145 NSRSs histopathologically diagnosed with lung cancer from Shanghai pulmonary hospital affiliated to Tongji University from January 2014 to January 2015, and detected the mutation status of EGFR, KRAS, BRAF, EML4-ALK and ROS1. The statistical analysis was also performed between SRSs and NSRSs. The results showed that there were no significant differences in age, sex and histological type. The grade presented significant results. More patients were grade I in surgical resection group, however, the patients in non surgical resection group was most of grade III and IV. As the development of early diagnosis of lung cancer and the use of computed tomography (CT), more and more lung cancer patients were detected in early stage, so these patients had the chance to resect the tumor according to surgical operation. Our results showed that more patients in early grade. The patients who are suffered with advanced lung cancer lose the opportunity of surgical resection, and could only use biopsy samples to detect the mutations of driver genes, so more advanced lung cancer patients were present in non surgical resection groups. In histological type analysis, there were no significant differences of age between SRSs and NSRSs. The sex showed the similar results expect in ADE group, more females in surgical resection group, and more male in non surgical resection group. General speaking, there was no significant difference between the two groups samples we collected, and could use to analysis the difference of driver gene mutations in the two groups.
Mutations in EGFR gene were firstly described to have the significant association with the treatment of advanced NSCLC in 2004 (11,12). The EGFR tyrosine kinase inhibitors (TKIs), such as erlotinib and gefitinib, have high sensitivity to EGFR mutation-positive tumors compared to platinum-based chemotherapy, and as the first-line treatment of lung cancer patients harbored EGFR mutation (13-15), especially for never-smokers or former light smoking Asian female patients with ADC (16). Recently, the fusion gene comprising of the echinoderm microtubule-associated protein-like 4 (EML4) gene and the ALK gene, has been reported in a subset of advanced NSCLC cases (17). Crizotinib, an inhibitor of c-MET, has high activity in the patients of lung cancer harbored EML4-ALK fusion (18), and also as the first-line treatment of lung cancer.
Many investigations showed that the target therapy only sensitive to target gene mutation, so to perform the target therapy as the first-line treatment, mutation testing should be firstly carried out to select optimal treatment. EGFR mutations of deletions in exon 19 and L858R point mutation in exon 21 are the most sensitivity mutations with a clear benefit from the first-line EGFR TKIs treatment (19). The L858R point mutation rate is higher than 19-del, the L858R is about 21%, and 19-del is about 19% in previous report (20). Our study showed the similar results, the 19-del is 17.9% in surgical resection group and 21.9% in non surgical resection group, L858R is 20.49 in surgical resection group and 23.39% in non surgical resection group. No significant difference was found between the two groups. Other mutations also presented no significant difference between the two groups. T790M point mutation in exon 20 and insertions in exon 20 could contribute to resistance to EGFR TKIs treatment, and the mutation rate is rare (21). Our study only detected 1.11% of insertion of exon 20 and less than 1% of T790M.
The testing of KRAS mutations was important because mutations in KRAS were also resistant to EGFR TKIs treatment. The KRAS mutations occurred about 21% in westerner, but much lower in Asian (about 5-11%) (22). Our results showed 8.39% and 7.04% in surgical resection group and non surgical resection group, respectively. There was no significant difference between the two groups. EML4-ALK fusion was about 4-7% in NSCLC (22), our study showed about 5% in both groups, and also presented no significant difference in the two groups. BRAF and ROS1 showed the same no significant difference results.
In conclusion, the surgical resection group and non surgical resection group analyzed in this study showed no significant differences both in clinical features and mutation status of EGFR, KRAS, BRAF, EML4-ALK and ROS1. The results suggested that the biopsy samples could use to detected driver gene mutation, and guide the clinical treatment of NSCLC.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Zhou C. Lung cancer molecular epidemiology in China: recent trends. Transl Lung Cancer Res 2014;3:270-9. [PubMed]
- Ryu JS, Memon A, Lee SK. ERCC1 and personalized medicine in lung cancer. Ann Transl Med 2014;2:32. [PubMed]
- Tang ER, Schreiner AM, Pua BB. Advances in lung adenocarcinoma classification: a summary of the new international multidisciplinary classification system (IASLC/ATS/ERS). J Thorac Dis 2014;6:S489-501. [PubMed]
- Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol 2015;33:861-9. [PubMed]
- Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 2011;24:653-64. [PubMed]
- Russell PA, Wainer Z, Wright GM, et al. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol 2011;6:1496-504. [PubMed]
- Aggarwal C. Targeted therapy for lung cancer: present and future. Ann Palliat Med 2014;3:229-35. [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [PubMed]
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [PubMed]
- Yang CF, D'Amico TA. Open, thoracoscopic and robotic segmentectomy for lung cancer. Ann Cardiothorac Surg 2014;3:142-52. [PubMed]
- Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [PubMed]
- Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 2004;101:13306-11. [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [PubMed]
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [PubMed]
- Antoniu SA. Crizotinib for EML4-ALK positive lung adenocarcinoma: a hope for the advanced disease? Evaluation of Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363(18):1693-703. Expert Opin Ther Targets 2011;15:351-3. [PubMed]
- Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361:958-67. [PubMed]
- Wang X, Wang G, Hao Y, et al. A comparison of ARMS and mutation specific IHC for common activating EGFR mutations analysis in small biopsy and cytology specimens of advanced non small cell lung cancer. Int J Clin Exp Pathol 2014;7:4310-6. [PubMed]
- Ayoola A, Barochia A, Belani K, et al. Primary and acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer: an update. Cancer Invest 2012;30:433-46. [PubMed]
- Dearden S, Stevens J, Wu YL, et al. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Ann Oncol 2013;24:2371-6. [PubMed]


