Beware the ‘raised right hemidiaphragm’ in a female patient with previous pneumothorax surgery: liver herniation through a massive endometrosis-related diaphragmatic fenestration
Introduction
Persistent pain and a raised hemi-diaphragm after surgery are often assumed to be iatrogenic and treated empirically. We report a case of massive diaphragmatic fenestration with visceral herniation in a patient with previous surgery for catamenial pneumothorax, mimicking these post-operative sequelae.
Case report
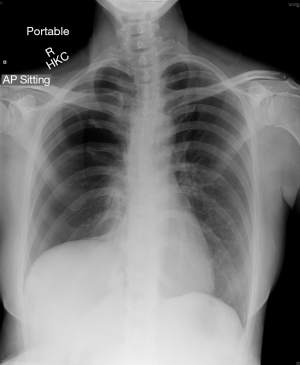
A non-smoking 44-year-old female patient presented with a recurrent right-sided pneumothorax. Nine years previously, she had received right lung bullectomy and pleurodesis via a video-assisted thoracic surgery (VATS) approach at another hospital for right-side pneumothorax. Although it was initially thought that she had primary pneumothorax, a diaphragm lesion intra-operatively, and histological analysis of this confirmed endometriosis. The patient was not given any endometriosis-specific treatment afterwards. Post-operatively, she complained of persistent right lower chest pain which was managed as post-operative neuropathic pain (1). However, the pain responded poorly to conventional and neuropathy-specific analgesia (pregabalin), and persisted throughout the intervening 9 years. Chest X-ray (CXR) also showed a “persistently elevated right diaphragm”, which was attributed to possible phrenic nerve palsy as a result of the surgery by the other hospital.
On presentation to our hospital presented with a recurrent right-sided pneumothorax now, CXR showed a large circumferential pneumothorax with no obvious inter-pleural adhesion. This CXR was again suggestive of an elevated right hemidiaphragm (Figure 1).
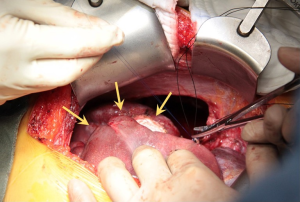
It was decided to offer her redo surgical pleurodesis in view of the extent of her recurrent pneumothorax. Initial exploration was performed using the standard right 2-port VATS technique. Intraoperatively, a 1.1 cm right apical lung bulla was found and staple-excised. However, much more strikingly, it was noted that the right liver had completely herniated via a large (10 cm × 9 cm) fenestration in the center of right hemidiaphragm (Figure 2). There were no tissue or membrane coverings over the fenestration, and the liver itself looked normal. The appearance was compatible with classic examples of endometriosis-related diaphragmatic fenestrations (2-4), but no previous cases to our knowledge have resulted in such a massive defect with such gross liver herniation into the right chest. In view of the extent of the defect, the operation was converted to a combined right limited thoracotomy plus right subcostal laparotomy approach. The right liver was mobilized and reduced into the abdomen via the hernia defect, and the latter was then repaired with a non-absorbable patch (Gore Dualmesh, Gore-Tex, Flagstaff, Ariz, USA) anchored with continuous 2-0 polypropylene sutures (Figure 3). Mechanical pleurodesis using abrasion to entire lateral chest wall parietal pleura was performed. One chest drain was inserted and −15 cmH2O suction was applied.
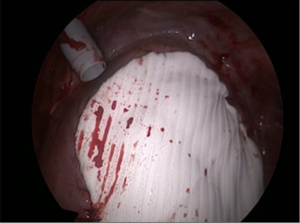
The patient had an uneventful post-operative recovery. The chest drain duration was 2 days, and she was discharged on postoperative day 4. Postoperative CXR showed restoration of the normal contour of the right hemidiaphragm. Pleural biopsies taken during surgery confirmed the presence of endometriosis. Her endometriosis was managed with regular intramuscular injection of gonadotropin-releasing hormone (GnRH) analogues on the advice of the gynecologists. She has remained free from recurrence of pneumothorax 15 months after the operation, and the right lower chest pain that had troubled her for the past 9 years had also fully resolved.
Discussion
Diaphragmatic fenestration is known to be associated with pulmonary endometriosis (2-10). However, endometriosis-related diaphragmatic fenestrations with abdominal visceral herniation is extremely rare. Only seven cases have been reported in the English literature (Table 1) (2-8), with the largest previously documented case having a diameter of 4 cm (2). To our knowledge, our patient reported here represents the most extreme case of diaphragmatic fenestration associated with catamenial pneumothorax yet reported in the literature—in terms of both fenestration size and extent of visceral herniation. Our case was particularly clinically significant in that it mimicked two common post-operative complications in thoracic surgery: post-thoracotomy/VATS pain and phrenic nerve palsy.
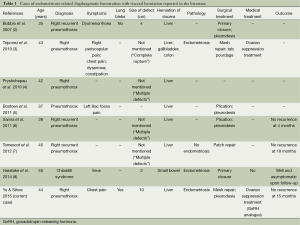
Full table
Persistent chest wall pain and paresthesia is a recognized complication after thoracic surgery, especially after traditional open thoracotomy. The advent of VATS for many thoracic surgical procedures has led to significant reduction in postoperative pain, but not in its elimination (11). Modern studies have shown that the reason for this may lie in the multifactorial nature of post-VATS pain (1,11). Although VATS reduces nociceptive and inflammatory components of postoperative pain, intercostal neuropathy can still remain leading to paresthesia. This multifactorial nature of post-VATS pain partly explains the inconsistent results of pain control with current modalities (e.g., oral analgesics, epidural/paravertebral infusions of local anesthetics, nerve blocks, cryotherapy etc.). As a specific treatment of neuropathy, gabapentin has now been established to be a safe, well-tolerated, and effective therapy for patients with persistent neuropathic pain following thoracic operations (1). In our patient, the previous hospital had managed her according to this line of thought using pregabalin—which is similar to gabapentin. However, no success was achieved even 9 years after the operation—which is a long time even for neuropathic sequelae. Our experience with this patient leads us to advise that failure to respond to conventional and even neuropathy-specific analgesia should raise suspicion of other causes of chronic chest pain.
The other suspected complication in our patient was a diaphragmatic hernia, suggested by the appearance of an “elevated hemidiaphragm” on CXR. Intra-operative physical, electrical or thermal trauma to the phrenic nerve may sometimes cause palsy, a well-recognized complication in any thoracic operation (12). It was not surprising that surgeons from the previous hospital believed this to have occurred based on the CXR appearance after the original operation. However, of all the operations in the human chest, pneumothorax surgery is rarely (if ever) associated with phrenic palsy. Bleb resections or bullectomies are very rarely performed in the vicinity of the phrenic nerve, and even mechanical pleurodesis or parietal pleurectomy tend to avoid the mediastinal pleura where the phrenic nerve runs. Our experience with this patient suggests that the appearance of an “elevated diaphragm” on CXR should not be automatically assumed to be due to phrenic injury—especially with procedures such as pneumothorax surgery which rarely involve the mediastinum. In such cases, other possible causes of the CXR change should be carefully considered.
In our patient, it is possible that the failure to treat the underlying endometriosis after the first operation contributed to both the subsequent recurrence of pneumothorax and the progressive enlargement of the diaphragmatic defect to such a massive degree. According to the literature, treatment with either surgery alone or hormonal therapy (e.g., GnRH analogues) alone for catamenial pneumothorax were both associated with high recurrence rates [surgery alone: 32% in 32 months (9); hormonal therapy alone: 60% in 12 months (10)]. Therefore, it has been suggested that a ‘combined’ or ‘sequential’ approach using both modalities should be the best treatment (10), in that the hormonal therapy could inactivate any residual endometriotic tissue left in the chest cavity after surgery. A recent case series of 12 patients demonstrated no recurrence on follow-up if surgical pleurodesis was followed immediately by GnRH analogues for 6-12 months (13). Because of the observation of what had happened to this patient with surgery alone 9 years ago, we chose to add hormonal therapy after the second operation.
Conclusions
Our experience with this patient suggests that a combination of refractory and extraordinarily persistent chest pain plus an “elevated right hemidiaphragm” on CXR and following surgery for pneumothorax in a female patient should ring warning bells for clinicians. Catamenial pneumothorax should be investigated for (if not already confirmed), and a massive fenestration of right hemidiaphragm with liver herniation should be suspected amongst the differential diagnoses for the presentation.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Sihoe AD, Lee TW, Wan IY, et al. The use of gabapentin for post-operative and post-traumatic pain in thoracic surgery patients. Eur J Cardiothorac Surg 2006;29:795-9. [PubMed]
- Bobbio A, Carbognani P, Ampollini L, et al. Diaphragmatic laceration, partial liver herniation and catamenial pneumothorax. Asian Cardiovasc Thorac Ann 2007;15:249-51. [PubMed]
- Triponez F, Alifano M, Bobbio A, et al. Endometriosis-related spontaneous diaphragmatic rupture. Interact Cardiovasc Thorac Surg 2010;11:485-7. [PubMed]
- Pryshchepau M, Gossot D, Magdeleinat P. Unusual presentation of catamenial pneumothorax. Eur J Cardiothorac Surg 2010;37:1221. [PubMed]
- Bostoen S, Van Raemdonck D, Dooms C. Why a chest physician should be interested in abdominal pain. Acta Clin Belg 2011;66:376-8. [PubMed]
- Sanna S, Taurchini M, Monteverde M, et al. Catamenially recurring pneumothorax with partial liver herniation: a particular view. Respiration 2011;82:476-7. [PubMed]
- Tomescot A, Fabre D. Catamenial pneumothorax with multiple transdiaphragmatic hepatic herniations. Asian Cardiovasc Thorac Ann 2012;20:205. [PubMed]
- Haratake N, Yamazaki K, Shikada Y. Diaphragmatic hernia caused by heterotopic endometriosis in Chilaiditi syndrome: report of a case. Surg Today 2014. [Epub ahead of print]. [PubMed]
- Alifano M, Jablonski C, Kadiri H, et al. Catamenial and noncatamenial, endometriosis-related or nonendometriosis-related pneumothorax referred for surgery. Am J Respir Crit Care Med 2007;176:1048-53. [PubMed]
- Alifano M, Trisolini R, Cancellieri A, et al. Thoracic endometriosis: current knowledge. Ann Thorac Surg 2006;81:761-9. [PubMed]
- Sihoe AD, Au SS, Cheung ML, et al. Incidence of chest wall paresthesia after video-assisted thoracic surgery for primary spontaneous pneumothorax. Eur J Cardiothorac Surg 2004;25:1054-8. [PubMed]
- Ostrowska M, de Carvalho M. Prognosis of phrenic nerve injury following thoracic interventions: Four new cases and a review. Clin Neurol Neurosurg 2012;114:199-204. [PubMed]
- Attaran S, Bille A, Karenovics W, et al. Videothoracoscopic repair of diaphragm and pleurectomy/abrasion in patients with catamenial pneumothorax: a 9-year experience. Chest 2013;143:1066-9. [PubMed]


