Epidemiological aspects of obstructive sleep apnea
Introduction
Obstructive sleep apnea (OSA) is a highly prevalent disorder, characterised by recurrent episodes of upper airway obstruction occurring during sleep, and associated with recurrent cycles of desaturation and re-oxygenation, sympathetic over-activity and intra-thoracic pressure changes, leading to fragmentation of sleep and consequent daytime fatigue and sleepiness (1). OSA is associated with decreased quality of life (QOL), significant functional impairment, and increased risk of road traffic accidents. However, the relationship of OSA with medical co-morbidity, and in particular cardiovascular co-morbidity, is perhaps the area of most interest from a medical perspective (2). This review will discuss several epidemiological aspects of OSA, namely its prevalence, its close relationship with obesity, and its economic impact, alongside a discussion of population-level, epidemiological data evaluating the impact of OSA on cardiovascular and metabolic disease, and its emerging association with cancer.
Prevalence of OSA
When defined as repetitive upper airway obstruction during sleep, OSA is a very common disorder. Sleep apnea severity is usually assessed by the apnea-hypopnoea index (AHI) which is the number of complete (apneas) or incomplete (hypopneas) obstructive events per hour of sleep. Defining OSA as an AHI ≥5 events/hour in the Wisconsin Sleep Cohort, the prevalence of OSA was 24% in men and 9% in women aged 30-60 years of age (3). The prevalence of OSA with associated excessive daytime somnolence is approximately 3% to 7% in adult men and 2% to 5% in adult women (4). Prevalence estimates don’t vary significantly worldwide suggesting that OSA is as common in the developing world as in western society (5).
As will be discussed in greater detail below, OSA has a particularly intimate association with obesity (4). Therefore, as the global obesity epidemic unfolds, it may be expected that the incidence and prevalence of sleep disordered breathing will increase in parallel with this. When data from the Wisconsin sleep cohort study were re-examined with adjustment for current levels of overweight and obesity, a marked increase in the prevalence of OSA was observed (6). Based on these data, 34% of men and 17.4% of women between the ages of 30-70 would be expected to have an AHI ≥5, which would be associated with excessive daytime sleepiness in 14% of men and 5% of women. Emerging data from Europe suggest that the societal prevalence of the disorder may be even greater when modern diagnostic techniques are used: a community-based Swiss study of over 2,000 subjects diagnosed moderate-severe OSA (i.e., an AHI ≥15) in 23.4% and 49% of female and male subjects, respectively (7), potentially alarming findings which need to be confirmed in other studies and other population groups.
OSA and obesity
Obesity, especially central adiposity, is consistently recognised as one of the strongest risk factors for OSA. Given the worsening modern pandemic of obesity in western society, the prevalence of OSA is likely to increase further (4). In the Wisconsin Sleep Cohort study, weight gain over a 4-year period was an important predictor of OSA progression; a 10% increase in body weight conferred a 32% increase in AHI and a 6-fold increase in the risk of developing moderate-severe OSA (8). In the Sleep Heart Health Study, a multi-centre epidemiologic cohort study of cardiovascular correlates of OSA in middle-aged and older Americans, weight gain of 10 kilograms over a 5-year period conferred a 5.2- and 2.5-fold increase in the likelihood of increasing the AHI by 15 events per hour in men and women respectively (9). OSA is present in 41% of patients with a body mass index (BMI) greater than 28 and the prevalence can be as high as 78% in patients referred for bariatric surgery (10,11).
OSA is marked by repetitive collapses of the upper airway during sleep that occur due to reduced airway dilator muscle tone. Obesity may alter the normal upper airway mechanics and contribute to the pathophysiology of OSA in a number of ways (4). Parapharyngeal fat deposition can result in a reduction in calibre and a change in shape of the upper airway promoting collapsibility (12,13). Obesity is associated with a reduction in lung volumes, especially functional residual capacity, contributing to decreased tracheal tug, a decrease in upper airway size and increased airflow resistance (14). Leptin is a hormone produced by adipocytes in proportion to their triglyceride content and involved in the suppression of appetite (15). However, it also acts on the central respiratory centres to stimulate ventilation and leptin deficiency has been associated with hypoventilation (16,17). Obesity is characterised by central leptin resistance and there is blunting of the response to hypercapnia leading to worsening of hypercapnia and impairment of arousal from sleep during apneas (18).
It is postulated that the interaction between OSA and obesity is not unidirectional, and that OSA may also have an impact on the pathogenesis of obesity (19). It is well recognized that weight gain may be preceded by the onset of OSA symptoms (20), and reduced physical activity with an associated reduction in energy expenditure due to excessive daytime somnolence in OSA can promote weight gain (21). Calorie intake in OSA patients may also be higher (22). Sleep deprivation is a feature of OSA and sleep curtailment is linked with obesity, particularly in pediatric populations (23), potentially through alterations in the hormonal regulation of diet. Finally a number of studies have demonstrated elevated leptin levels in OSA patients compared to weight-matched controls and a decrease in leptin levels following continuous positive airway pressure (CPAP) therapy (24,25), suggesting that OSA may influence leptin metabolism independently of changes in weight (26).
Weight loss should be recommended for all overweight or obese patients with OSA, as it may confer not only a benefit in reducing OSA severity, but also a positive impact on other obesity-related diseases such as type 2 diabetes mellitus (T2DM). Patients with more severe OSA derive more benefit from weight loss than those with milder disease (27). Several studies have explored weight loss as a therapeutic option for OSA and approaches evaluated include behavioral methods (dietary modification and exercise), pharmacological methods and bariatric surgery (28). The results for behavioral modification have been mixed. Johansson reported a significant reduction in AHI to such an extent that 17% were cured, no longer requiring CPAP (27). Results from other studies showed no change in AHI however (29,30). Sibutramine, an oral anorexiant, has been evaluated in a number of studies in OSA patients, but any positive benefit it may have has now been superseded by concerns related to increased cardiovascular morbidity (nonfatal myocardial infarction and stroke) associated with its use (28).
Studies on the effects of bariatric surgery appear promising. In a Swedish case-control longitudinal study of more than 3,400 obese patients, the average fall in BMI was −9.7±5 kg.m-2 compared to 0±3 kg.m-2 in the control group (31). There was also a marked improvement in OSA symptoms and a lower 2-year incidence of T2DM and hypertriglyceridemia. Laparoscopic adjustable gastric banding in a cohort of severely obese patients with moderate to severe OSA resulted in significant weight loss and a decrease in AHI when evaluated at 17.7±10.0 months after surgery (32). However, the majority of patients will still have residual OSA after surgery and care should be taken to ensure CPAP is not inappropriately discontinued (33,34).
OSA and age
The prevalence of OSA increases with age in adults (5). This age-related increase in prevalence may be attributable to parapharyngeal fat deposition, lengthening of the soft palate and changes in other anatomic parapharyngeal structures (35). The Sleep Heart Health Study demonstrated that the prevalence of OSA plateaued after the age of 60 years (36), and the increased risk of all-cause and cardiovascular mortality associated with OSA is mainly limited to middle-aged adults, especially men (37). Some researchers have suggested that the mortality risk with sleep apnea may even decrease in the elderly as a result of preconditioning cardioprotective adaptations to chronic intermittent hypoxia (38). Elderly patients with and without excessive daytime somnolence may represent different phenotypes that explain the conflicting data on mortality risk with increasing age in OSA; the presence of concomitant excessive daytime somnolence in OSA patients may increase the risk of mortality whereas the same severity of OSA without symptoms may not (39).
OSA and sex
It is well recognized that there is a higher prevalence of OSA in men than women, with most population-based studies demonstrating a 2- to 3-fold higher prevalence of OSA in men (4). Men are also more likely to be referred for clinical assessment for OSA (40), perhaps because physicians appear to have a higher index of suspicion for considering the disorder in men. This tendency may contribute to the underdiagnosis of OSA in women in clinical practice, a bias which may be compounded by the fact that women often do not present with the classical symptoms of OSA (loud snoring, witnessed apneas and excessive daytime somnolence) but instead may complain of poor energy levels and fatigue (41). Furthermore, the female bed partners of male patients may be more likely to perceive and report snoring or nocturnal breathing abnormalities than male bed partners of female patients.
Sex hormones may also play an important role in the pathogenesis of OSA. OSA is more prevalent in post-menopausal women than pre-menopausal women, and hormone replacement therapy in post-menopausal women may protect against the disorder (42,43).
Genetic aspects of OSA
Given its complexity as a disorder with multiple predisposing factors, the likelihood of a single governing genetic factor causing OSA is extremely low. That said, it is thought that up to 40% of the risk of OSA is genetically predisposed (44). The prevalence of OSA in first-degree relatives of patients with OSA ranges from 22-84% with an odds ratio (OR) of a first-degree relative having OSA ranging from 2 to 46 (45).
Anatomic risk factors for OSA, such as obesity and upper airway soft tissue structure, demonstrate familial aggregation. Obesity often persists from early life to late middle age and twin studies on Caucasian populations demonstrate a heritability estimate of 57-86% for the trend of BMI from early adulthood to late middle age (46). A case-control study in a Scottish cohort identified a strong familial component to OSA and suggested that differences in facial structure were more important than obesity in this regard (47). The lateral pharyngeal wall volume, tongue volume and total upper airway soft tissue volume have a significant level of heritability after adjusting for sex, age, ethnic background, craniofacial properties and neck fat deposition.
A number of candidate gene associations have been investigated in OSA including different alleles for apolipoprotein E4 (ApoE4), tumour necrosis factor (TNF), and angiotensin-converting enzyme (ACE) but only one TNF polymorphism (TNFA rs1800629) was significantly associated with OSA under an allele frequency model (48).
Economic aspects of OSA
OSA has a significant economic impact on healthcare systems and society. OSA-related healthcare cost includes the direct costs of OSA diagnosis and treatment and the indirect costs of associated conditions (obesity, diabetes) and sequelae (cardiovascular disease, depression). Patients with sleep disorders are less productive workers than those without sleep disorders and have a higher level of absenteeism and decreased productivity at work due to fatigue (49). Occupational injuries are also more common in patients with OSA (50).
Several studies have shown that QOL is adversely affected in patients with OSA and that it improves with therapy (51-53). The bed partner of a patient with untreated OSA may also suffer from disrupted sleep because of the patient’s snoring, gasping or witnessed apneas. The QOL of bed partners also improves significantly when OSA is treated (54,55).
Untreated patients with OSA are at an increased risk of motor vehicle accidents (MVAs). Patients with OSA have a 2- to 10-fold increased risk of MVAs and the severity of accident may be greater compared to controls (56,57). OSA-related MVAs in the USA were estimated to involve 800,000 drivers and cost $15.9 billion annually (58). Treatment with CPAP therapy reduces this risk in compliant patients to that of controls (59).
The results of cost-effectiveness analyses support the utilization of CPAP therapy in patients with moderate to severe OSA relative to other commonly accepted medical interventions. It is estimated that it costs $2,000—11,000 per quality-adjusted life year (QALY) over 5 years to treat moderate to severe OSA in a sleep clinic population compared to $5,000—19,000 for breast cancer screening and $10,000—57,000 for hypertension control (5).
Epidemiology of co-morbidity in OSA
OSA and cardiovascular disease
OSA and hypertension
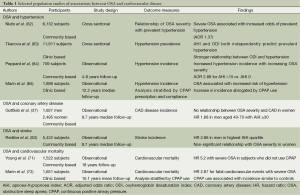
The most robust evidence supporting an independent role for OSA in promoting adverse cardiovascular outcomes is to be found in studies addressing its relationship with hypertension (1). Following on from a number of studies where snorers were more likely to also have hypertension than might otherwise be expected (60), a series of clinical and epidemiological studies identified a dose-response relationship between OSA severity and the likelihood of prevalent hypertension (61). For example, a cross sectional analysis of over 6,000 North American subjects enrolled in the community-based Sleep Heart Health Study showed that subjects with severe sleep disordered breathing had an OR of 1.37 [95% confidence interval (CI): 1.03-1.83] for prevalent hypertension following adjustment for confounding factors when compared to those with no OSA (62). Recent European data underline this association, and suggest that intermittent hypoxemia may be a key factor contributing to co-existent hypertension—among 11,900 participants in the multi-national the European Sleep Apnea Cohort (ESADA) study, OSA severity indices, and in particular the oxyhemoglobin desaturation index, were strong independent predictors of hypertension (63).
Subsequent longitudinal studies have provided convincing evidence that OSA contributes to an increased risk of clinically-relevant hypertension. Landmark findings among participants in the Wisconsin Sleep Cohort study demonstrated a strikingly increased propensity for the development of incident hypertension in subjects with sleep disordered breathing (64). In an analysis of 709 subjects, the presence severe OSA at enrollment conferred a nearly threefold risk of being diagnosed with hypertension over a four-year follow-up period, independently of the effects of age, obesity and smoking history. More recent data from Spain have confirmed this relationship (65).
OSA and coronary artery disease (CAD)
Data from clinic and community based studies generally suggest that CAD is highly prevalent in OSA cohorts (66), and vice versa that subjects with CAD are more likely to also have sleep disordered breathing, even allowing for the impact of obesity and other confounding factors (67). For example, in over 6,000 participants in the Sleep Heart Health Study, the burden of self-reported CAD among these subjects increased from 9% in the lowest AHI quartile to 19% in the highest, a relationship that survived adjustment for confounding variables (68).
It is less clear if the presence and severity of OSA actually serve as independent predictors of subsequent CAD, however. While data from relatively small, but well-conducted studies of hospital patients suggest a robust dose-response relationship between the two (69), these data have not been reproduced to the same degree at a population level. A relatively modest relationship between OSA and CAD incidence was seen in a community-based study of 4,422 (56.4% female) North American subjects followed for a median of 8.7 years (37). Within this cohort, severe OSA predicted an increased risk of developing symptomatic CAD, but only in men aged 70 or less (HR 1.68; 95% CI: 1.02-2.76). However, another analysis of this cohort has shown severe OSA to be an independent predictor of death, and in particular death related to CAD (70). While this relationship was again strongest in men under 70 years of age (adjusted HR 2.09; 95% CI: 1.31-3.33), it was nonetheless seen across the entire study population (adjusted HR 1.46; 95% CI: 1.14-1.86), with similar findings seen at eighteen year follow-up in the Wisconsin Sleep Cohort study (71).
Were OSA playing a causative role in driving cardiovascular morbidity and mortality, CPAP therapy might be expected to lead to measurable reductions in adverse cardiac outcomes. In a study of 1,652 subjects attending a Spanish sleep laboratory, cardiovascular outcomes were assessed over an average of 10.1 years (72). Those with untreated severe OSA (14.2%) were more likely to die of cardiovascular disease (adjusted OR 2.87; 95% CI: 1.17-7.51) or experience cardiovascular morbidity (adjusted OR 3.17; 95% CI: 1.12-7.51) compared with healthy non-apneic subjects. In contrast, no significant increased risk of cardiac death was seen in OSA patients who had been successfully commenced on CPAP (adjusted OR 1.05; 95% CI: 0.39-2.21). As yet there are no published data from large-scale randomised trials in this area, although a number of potentially important studies with this goal are ongoing.
OSA and heart failure
Obstructive sleep apnea is highly prevalent in heart failure patients, and is independently associated with increased prevalence of clinically overt heart failure (73). In a cross sectional analysis of the Sleep Heart Health study, severe OSA was associated with increased likelihood of prevalent heart failure, with an adjusted OR of 2.20 (95% CI: 1.11-4.37) in the highest AHI quartile (68). Longitudinal follow-up in the same cohort identified a strong relationship between incident heart failure and AHI in men but not women (37). Following adjustment for demographic, anthropometric and clinical factors and medication usage, male participants with severe OSA at baseline had a 58% increased risk of developing heart failure over nearly nine years of follow-up. When present, OSA appears to lead to increased risk of mortality in heart failure cohorts (74).
OSA and cardiac arrhythmia
Subjects with OSA are at increased risk of atrial fibrillation (AF); in a nested case control study involving 566 participants in the Sleep Heart Health study, severe sleep disordered breathing was associated with a four-fold odds of prevalent AF, following adjustment for age, gender, obesity and the presence of CAD (adjusted OR 4.02; 95% CI: 1.03-15.74) (75). The presence of OSA predicts an increased likelihood of risk factors for AF, such as increased left atrial diameter (76), and furthermore is associated with an increased risk of recurrence of AF and failure of chemical cardioversion measures (77,78). Moreover, recurrence of AF becomes less likely with the initiation of CPAP treatment (77). Sleep disordered breathing is also an independent predictor of ventricular arrhythmia, particularly in subjects with heart failure (75).
OSA and cerebrovascular disease
Sleep disordered breathing is significantly more common in patients who have had a stroke or transient ischaemic attack (TIA) than in the general community, occurring in between 32-63% of stroke patients, and is associated with increased mortality and worse functional outcomes in these patients (79,80). Furthermore, cerebrovascular disease prevalence appears to increase with increasing OSA severity. Among 6,089 subjects in the Sleep Heart Health Study, 2.7% in the lowest AHI quartile had a prior stroke or TIA, compared with 5.3% in the most severe quartile, a relationship which persisted following adjustment for relevant confounders (adjusted OR 1.58; 95% CI: 1.02-2.46) (68). A number of prospective studies suggest that the presence and severity of sleep disordered breathing predicts incident stroke (81-84). For example, among 5,422 North American subjects followed for a median of 8.7 years, those in the most severe AHI quartile had an adjusted HR 2.86 (95% CI: 1.1-7.4) for stroke over the study period (83) (Table 1).
Full table
OSA and metabolic disease
The complex relationship between OSA, obesity and metabolic disease, and in particular insulin resistance, glucose intolerance and T2DM is the subject of a forthcoming review in Journal of Thoracic Disease, and will not be discussed in detail here. Briefly, it does appear that OSA severity has a direct bearing on metabolic health. For example, in a large study of over 6,000 subjects attending European sleep laboratories, severe OSA was associated with an almost twofold increase in likelihood (adjusted OR 1.87) of concomitant T2DM, even following adjustemnt for the confounding effects of obesity, age and other variables (85). Moreover, diabetic patients with severe OSA within this cohort had higher HbA1c levels, and were more likely to have poorly controlled T2DM than non-apneic diabetics. Similarly, in an historical cohort study of 8,678 Canadian sleep clinic patients, followed for a median time period of 67 months, a diagnosis of severe OSA conferred a 37% increase in risk for incident T2DM, following statistical adjustment for confounding factors (86).
Insulin resistance and glucose intolerance also appear to be more common in OSA populations. In a large North American community-based cohort, severity of nocturnal hypoxemia and the presence of at least moderately severe OSA were both associated with an increased odds of insulin resistance (87), while a study of over 5,000 non-diabetic European sleep clinic patients found that HbA1c levels increased in parallel with AHI, irrespective of the influence of obesity (88).
Once again, the ability of CPAP to make a meaningful difference to metabolic health in patients with OSA remains unproven. A number of well-conducted, if relatively small, randomised trials have generally failed to identify anything more than a minor impact from CPAP therapy on a range of metabolic variables (89-92).
OSA and malignancy
Sleep disordered breathing generates a molecular environment which is certainly markedly pro-atherogenic (93), but could also potentially promote the development of cancer. A recognition that OSA causes tissue hypoxia, systemic inflammation, oxidative stress and immune dysregulation (93), all factors associated with oncogenesis, lead a number of investigators to examine the effects of intermittent hypoxia in a rodent model of maligant melanoma. Data from these animal models suggested that exposure to IH may increase tumor progression, and appears to promote tumor metastasis (94,95).
A number of longitudinal studies have since suggested that an association between OSA, nocturnal hypoxemia and cancer may not be confined to rodents living in laboratories. In an analysis of 1,522 subjects enrolled in the community-based Wisconsin Sleep Cohort, severe sleep disordered breathing was associated with an almost five-fold risk of cancer death (96). Similarly, in a cohort of nearly 5,000 Spanish patients attending sleep clinics, severity of nocturnal hypoxemia predicted incident cancer, even following rigorous adjustment for confounding variables (97). Conversely, a large retrospective cohort study of Canadian sleep found that any relationship between OSA and cancer incidence could be attributed to conventional, established risk factors for malignancy (98); however, even within this population an independent association was seen between smoking-related cancer and severity of nocturnal hypoxemia. To date, there are no published data examining the effect of CPAP therapy on cancer outcomes, but potentially ground-breaking clinical trials are ongoing in this area.
Conclusions
OSA is an increasingly common disorder, with a particularly intimate relationship with obesity. OSA leads to impaired QOL, an increased risk of MVAs and impaired workplace performance. Furthermore, it contributes to development of clinically overt cardiovascular disease, and may also lead to increased risk of metabolic disease and cancer. Consequently, OSA represents a significant, evolving public health challenge in both the developed and developing world.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- McNicholas WT, Bonsignore MR. Management Committee of EU COST ACTION B26. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J 2007;29:156-78. [PubMed]
- Kent BD, Ryan S, McNicholas WT. Obstructive sleep apnea and inflammation: relationship to cardiovascular co-morbidity. Respiratory physiology & neurobiology 2011;178:475-81. [PubMed]
- Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230-5. [PubMed]
- Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 2008;5:136-43. [PubMed]
- Kapur VK. Obstructive sleep apnea: diagnosis, epidemiology, and economics. Respir Care 2010;55:1155-67. [PubMed]
- Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013;177:1006-14. [PubMed]
- Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 2015;3:310-8. [PubMed]
- Peppard PE, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000;284:3015-21. [PubMed]
- Newman AB, Foster G, Givelber R, et al. Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern Med 2005;165:2408-13. [PubMed]
- Vgontzas AN, Tan TL, Bixler EO, et al. Sleep apnea and sleep disruption in obese patients. Arch Intern Med 1994;154:1705-11. [PubMed]
- Lopez PP, Stefan B, Schulman CI, et al. Prevalence of sleep apnea in morbidly obese patients who presented for weight loss surgery evaluation: more evidence for routine screening for obstructive sleep apnea before weight loss surgery. Am Surg 2008;74:834-8. [PubMed]
- Schwartz AR, Patil SP, Squier S, et al. Obesity and upper airway control during sleep. J Appl Physiol (1985) 2000;108:430-5. [PubMed]
- Shelton KE, Woodson H, Gay S, et al. Pharyngeal fat in obstructive sleep apnea. Am Rev Respir Dis 1993;148:462-6. [PubMed]
- Steier J, Lunt A, Hart N, et al. Observational study of the effect of obesity on lung volumes. Thorax 2014;69:752-9. [PubMed]
- Münzberg H, Morrison CD. Structure, production and signaling of leptin. Metabolism: clinical and experimental 2015;64:13-23. [PubMed]
- Atwood CW. Sleep-related hypoventilation: the evolving role of leptin. Chest 2005;128:1079-81. [PubMed]
- Kalra SP. Central leptin insufficiency syndrome: an interactive etiology for obesity, metabolic and neural diseases and for designing new therapeutic interventions. Peptides 2008;29:127-38. [PubMed]
- Campo A, Fruhbeck G, Zulueta JJ, et al. Hyperleptinaemia, respiratory drive and hypercapnic response in obese patients. Eur Respir J 2007;30:223-31. [PubMed]
- Ryan S, Crinion SJ, McNicholas WT. Obesity and sleep-disordered breathing--when two 'bad guys' meet. QJM 2014;107:949-54. [PubMed]
- Phillips BG, Hisel TM, Kato M, et al. Recent weight gain in patients with newly diagnosed obstructive sleep apnea. J Hypertens 1999;17:1297-300. [PubMed]
- O'Driscoll DM, Turton AR, Copland JM, et al. Energy expenditure in obstructive sleep apnea: validation of a multiple physiological sensor for determination of sleep and wake. Sleep Breath 2013;17:139-46. [PubMed]
- Beebe DW, Miller N, Kirk S, et al. The association between obstructive sleep apnea and dietary choices among obese individuals during middle to late childhood. Sleep Med 2011;12:797-9. [PubMed]
- Spiegel K, Knutson K, Leproult R, et al. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol (1985) 2005;99:2008-19. [PubMed]
- Chen X, Niu X, Xiao Y, et al. Effect of Continuous Positive Airway Pressure on Leptin Levels in Patients with Obstructive Sleep Apnea: A Meta-analysis. Otolaryngol Head Neck Surg 2015;152:610-618. [PubMed]
- Phillips BG, Kato M, Narkiewicz K, et al. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am J Physiol Heart Circ Physiol 2000;279:H234-7. [PubMed]
- Ong CW, O'Driscoll DM, Truby H, et al. The reciprocal interaction between obesity and obstructive sleep apnoea. Sleep Med Rev 2013;17:123-31. [PubMed]
- Johansson K, Hemmingsson E, Harlid R, et al. Longer term effects of very low energy diet on obstructive sleep apnoea in cohort derived from randomised controlled trial: prospective observational follow-up study. BMJ 2011;342:d3017. [PubMed]
- Cowan DC, Livingston E. Obstructive sleep apnoea syndrome and weight loss: review. Sleep Disord 2012;2012:163296.
- Barnes M, Goldsworthy UR, Cary BA, et al. A diet and exercise program to improve clinical outcomes in patients with obstructive sleep apnea--a feasibility study. J Clin Sleep Med 2009;5:409-15. [PubMed]
- Nerfeldt P, Nilsson BY, Mayor L, et al. A two-year weight reduction program in obese sleep apnea patients. J Clin Sleep Med 2010;6:479-86. [PubMed]
- Grunstein RR, Stenlof K, Hedner JA, et al. Two year reduction in sleep apnea symptoms and associated diabetes incidence after weight loss in severe obesity. Sleep 2007;30:703-10. [PubMed]
- Dixon JB, Schachter LM, O'Brien PE. Polysomnography before and after weight loss in obese patients with severe sleep apnea. Int J Obes (Lond) 2005;29:1048-54. [PubMed]
- Greenburg DL, Lettieri CJ, Eliasson AH. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med 2009;122:535-42. [PubMed]
- Lettieri CJ, Eliasson AH, Greenburg DL. Persistence of obstructive sleep apnea after surgical weight loss. J Clin Sleep Med 2008;4:333-8. [PubMed]
- Malhotra A, Huang Y, Fogel R, et al. Aging influences on pharyngeal anatomy and physiology: the predisposition to pharyngeal collapse. Am J Med 2006;119:72.e9-14.
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002;165:1217-39. [PubMed]
- Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation 2010;122:352-60. [PubMed]
- Lavie P, Lavie L. Unexpected survival advantage in elderly people with moderate sleep apnoea. J Sleep Res 2009;18:397-403. [PubMed]
- Gooneratne NS, Richards KC, Joffe M, et al. Sleep disordered breathing with excessive daytime sleepiness is a risk factor for mortality in older adults. Sleep 2011;34:435-42. [PubMed]
- Evans J, Skomro R, Driver H, et al. Sleep laboratory test referrals in Canada: sleep apnea rapid response survey. Can Respir J 2014;21:e4-10. [PubMed]
- Young T, Evans L, Finn L, et al. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997;20:705-6. [PubMed]
- Shahar E, Redline S, Young T, et al. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med 2003;167:1186-92. [PubMed]
- Wesström J, Ulfberg J, Nilsson S. Sleep apnea and hormone replacement therapy: a pilot study and a literature review. Acta Obstet Gynecol Scand 2005;84:54-7. [PubMed]
- Kent BD, Ryan S, McNicholas WT. The genetics of obstructive sleep apnoea. Curr Opin Pulm Med 2010;16:536-42. [PubMed]
- Redline S, Tishler PV. The genetics of sleep apnea. Sleep Med Rev 2000;4:583-602. [PubMed]
- Silventoinen K, Kaprio J. Genetics of tracking of body mass index from birth to late middle age: evidence from twin and family studies. Obes Facts 2009;2:196-202. [PubMed]
- Mathur R, Douglas NJ. Family studies in patients with the sleep apnea-hypopnea syndrome. Ann Intern Med 1995;122:174-8. [PubMed]
- Varvarigou V, Dahabreh IJ, Malhotra A, et al. A review of genetic association studies of obstructive sleep apnea: field synopsis and meta-analysis. Sleep 2011;34:1461-8. [PubMed]
- Sherman B. Obstructive sleep apnea and health benefits purchasing: an employer perspective. J Clin Sleep Med 2013;9:187-9. [PubMed]
- AlGhanim N, Comondore VR, Fleetham J, et al. The economic impact of obstructive sleep apnea. Lung 2008;186:7-12. [PubMed]
- Smith IE, Shneerson JM. Is the SF 36 sensitive to sleep disruption? A study in subjects with sleep apnoea. J Sleep Res 1995;4:183-8. [PubMed]
- Jenkinson C, Stradling J, Petersen S. Comparison of three measures of quality of life outcome in the evaluation of continuous positive airways pressure therapy for sleep apnoea. J Sleep Res 1997;6:199-204. [PubMed]
- Gall R, Isaac L, Kryger M. Quality of life in mild obstructive sleep apnea. Sleep 1993;16:S59-61. [PubMed]
- Parish JM, Lyng PJ. Quality of life in bed partners of patients with obstructive sleep apnea or hypopnea after treatment with continuous positive airway pressure. Chest 2003;124:942-7. [PubMed]
- Kiely JL, McNicholas WT. Bed partners' assessment of nasal continuous positive airway pressure therapy in obstructive sleep apnea. Chest 1997;111:1261-5. [PubMed]
- Ellen RL, Marshall SC, Palayew M, et al. Systematic review of motor vehicle crash risk in persons with sleep apnea. J Clin Sleep Med 2006;2:193-200. [PubMed]
- Ayas NT, FitzGerald JM, Fleetham JA, et al. Cost-effectiveness of continuous positive airway pressure therapy for moderate to severe obstructive sleep apnea/hypopnea. Arch Intern Med 2006;166:977-84. [PubMed]
- Sassani A, Findley LJ, Kryger M, et al. Reducing motor-vehicle collisions, costs, and fatalities by treating obstructive sleep apnea syndrome. Sleep 2004;27:453-8. [PubMed]
- George CF. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax 2001;56:508-12. [PubMed]
- Lugaresi E, Cirignotta F, Coccagna G, et al. Some epidemiological data on snoring and cardiocirculatory disturbances. Sleep 1980;3:221-4. [PubMed]
- Hedner J, Bengtsson-Bostrom K, Peker Y, et al. Hypertension prevalence in obstructive sleep apnoea and sex: a population-based case-control study. Eur Respir J 2006;27:564-70. [PubMed]
- Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000;283:1829-36. [PubMed]
- Tkacova R, McNicholas WT, Javorsky M, et al. Nocturnal intermittent hypoxia predicts prevalent hypertension in the European Sleep Apnoea Database cohort study. Eur Respir J 2014;44:931-41. [PubMed]
- Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000;342:1378-84. [PubMed]
- Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 2012;307:2169-76. [PubMed]
- Kent BD, Garvey JF, Ryan S, et al. Severity of obstructive sleep apnoea predicts coronary artery plaque burden: a coronary computed tomographic angiography study. Eur Respir J 2013;42:1263-70. [PubMed]
- Mooe T, Rabben T, Wiklund U, et al. Sleep-disordered breathing in men with coronary artery disease. Chest 1996;109:659-63. [PubMed]
- Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med 2001;163:19-25. [PubMed]
- Mooe T, Franklin KA, Holmstrom K, et al. Sleep-disordered breathing and coronary artery disease: long-term prognosis. Am J Respir Crit Care Med 2001;164:1910-3. [PubMed]
- Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009;6:e1000132. [PubMed]
- Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 2008;31:1071-8. [PubMed]
- Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046-53. [PubMed]
- Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet 2009;373:82-93. [PubMed]
- Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol 2007;49:1625-31. [PubMed]
- Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. American journal of respiratory and critical care medicine 2006;173:910-6. [PubMed]
- Drager LF, Bortolotto LA, Pedrosa RP, et al. Left atrial diameter is independently associated with arterial stiffness in patients with obstructive sleep apnea: potential implications for atrial fibrillation. Int J Cardiol 2010;144:257-9. [PubMed]
- Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003;107:2589-94. [PubMed]
- Monahan K, Brewster J, Wang L, et al. Relation of the severity of obstructive sleep apnea in response to anti-arrhythmic drugs in patients with atrial fibrillation or atrial flutter. Am J Cardiol 2012;110:369-72. [PubMed]
- Bassetti C, Aldrich MS. Sleep apnea in acute cerebrovascular diseases: final report on 128 patients. Sleep 1999;22:217-23. [PubMed]
- Good DC, Henkle JQ, Gelber D, et al. Sleep-disordered breathing and poor functional outcome after stroke. Stroke 1996;27:252-9. [PubMed]
- Arzt M, Young T, Finn L, et al. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med 2005;172:1447-51. [PubMed]
- Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005;353:2034-41. [PubMed]
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med 2010;182:269-77. [PubMed]
- Munoz R, Duran-Cantolla J, Martinez-Vila E, et al. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke 2006;37:2317-21. [PubMed]
- Kent BD, Grote L, Ryan S, et al. Diabetes mellitus prevalence and control in sleep-disordered breathing: the European Sleep Apnea Cohort (ESADA) study. Chest 2014;146:982-90. [PubMed]
- Kendzerska T, Gershon AS, Hawker G, et al. Obstructive sleep apnea and incident diabetes. A historical cohort study. Am J Respir Crit Care Med 2014;190:218-25. [PubMed]
- Punjabi NM, Shahar E, Redline S, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol 2004;160:521-30. [PubMed]
- Kent BD, Grote L, Bonsignore MR, et al. Sleep apnoea severity independently predicts glycaemic health in nondiabetic subjects: the ESADA study. Eur Respir J 2014;44:130-9. [PubMed]
- West SD, Nicoll DJ, Wallace TM, et al. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax 2007;62:969-74. [PubMed]
- Coughlin SR, Mawdsley L, Mugarza JA, et al. Cardiovascular and metabolic effects of CPAP in obese males with OSA. Eur Respir J 2007;29:720-7. [PubMed]
- Lam JC, Lam B, Yao TJ, et al. A randomised controlled trial of nasal continuous positive airway pressure on insulin sensitivity in obstructive sleep apnoea. Eur Respir J 2010;35:138-45. [PubMed]
- Hoyos CM, Sullivan DR, Liu PY. Effect of CPAP on the metabolic syndrome: a randomised sham-controlled study. Thorax 2013;68:588-9. [PubMed]
- Garvey JF, Taylor CT, McNicholas WT. Cardiovascular disease in obstructive sleep apnoea syndrome: the role of intermittent hypoxia and inflammation. Eur Respir J 2009;33:1195-205. [PubMed]
- Almendros I, Montserrat JM, Torres M, et al. Intermittent hypoxia increases melanoma metastasis to the lung in a mouse model of sleep apnea. Respir Physiol Neurobiol 2013;186:303-7. [PubMed]
- Almendros I, Montserrat JM, Ramirez J, et al. Intermittent hypoxia enhances cancer progression in a mouse model of sleep apnoea. Eur Respir J 2012;39:215-7. [PubMed]
- Nieto FJ, Peppard PE, Young T, et al. Sleep-disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med 2012;186:190-4. [PubMed]
- Campos-Rodriguez F, Martinez-Garcia MA, Martinez M, et al. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med 2013;187:99-105. [PubMed]
- Kendzerska T, Leung RS, Hawker G, et al. Obstructive sleep apnea and the prevalence and incidence of cancer. CMAJ 2014;186:985-92. [PubMed]


