Clinical characteristics of idiopathic pulmonary fibrosis patients with gender, age, and physiology staging at Okinawa Chubu Hospital
Introduction
Idiopathic pulmonary fibrosis (IPF) is a common, progressive fibrotic lung disease characterized by honeycombing on high-resolution computed tomography (HRCT) images (1-3). The median survival time of IPF patients is approximately 3 years (4) and the most common cause of death is progressive respiratory failure (5,6). Some IPF patients have a stable clinical course, whereas others have variable long-term courses and survival (7,8). Thus, predicting survival with IPF is quite important for patients.
For clinicians, many useful staging algorithms have provided useful information, such as for systemic sclerosis-associated interstitial lung disease (ILD) (9), lung cancer (10), and chronic obstructive lung disease (COPD) (11). A prediction model for IPF was proposed (12), although this is quite complicated for use in clinical practice. Recently, some staging systems that used simple variables were proposed for IPF, which included respiratory hospitalization or gender, age, and physiology (GAP) (13,14).
Kazerooni et al. proposed a CT scoring system for IPF (15). In addition, the latest IPF guidelines emphasize the utility of HRCT findings (16). From the clinical perspective, a simple staging system that is applicable to real world situations is desirable. However, the clinical and radiological characteristics of IPF according to GAP staging remain unknown.
Thus, in this study, we used the GAP staging system to determine the clinical characteristics, HRCT findings, and the most important predictors of mortality for IPF patients.
Materials and methods
IPF patients and recorded results
We retrospectively reviewed the medical records and chest HRCT images of IPF patients who visited our hospital from June 1, 2002 to December 31, 2012. And we chose IPF patients with diagnostic criteria of 2011 international IPF guideline (16). We recorded age, gender, smoking history, modified Medical Research Council (mMRC) dyspnea scale scores (17), dyspnea duration, pulmonary hypertension, and survival. We also recorded repeated mMRC evaluations that were made after 1 year.
We also reviewed the laboratory results for white blood cell (WBC) counts and lactate dehydrogenase (LDH), C-reactive protein (CRP), and Krebs von den Lungen-6 (KL-6) levels. In terms of physiological findings, we recorded vital capacity (VC), percent VC (% VC), forced vital capacity (FVC), percent FVC (% FVC), total lung capacity (TLC), percent TLC (% TLC), percent FEV1 (% FEV1), DLco, % DLco, DLco/alveolar volume (KCO), and percent KCO (% KCO). We also performed cardiac echograms each year to evaluate pulmonary arterial hypertension (PAH).
And the definition of PAH was over 35 mmHg of estimated systolic pulmonary arterial pressure, which was calculated by transtricuspid pressure gradient pressure plus right atrial pressure. Chest CT images were obtained with 1.5-mm-thick axial sections at 1-cm intervals throughout the thorax during the inspiratory phase. No oral or intravenous contrast material was administered. We selected three levels for image scoring. These were at the aortic arch, the carina, and 1 cm above the right diaphragm. We also evaluated ground glass opacity (GGO) and honeycombing. GGO was defined as increased opacity in the parenchyma without obscuring of any lung vessel.
Honeycombing was defined as a cluster of relatively thick-walled (1-3 mm) cysts that were 3-10 mm in diameter with multiple cysts that shared walls and a single layer of clustered subpleural cysts located in the periphery of the lung, thus ensuring that paraseptal emphysema was not present (18). Each lung lobe was scored on a scale of 0-5 (0, <5%, 5% to <25%, 25% to <50%, 50% to <75%, and >75% lobe involvement) for both GGO and honeycombing on the basis of the scoring system of Kazerooni et al. (15). The GGO and honeycombing scores for each lobe were averaged for an individual patient.
GAP staging system
For the GAP staging system, we reviewed % FVC and % DLco results. In addition, we assessed % FEV1, % TLC, and % KCO. We calculated the composite physiologic index (CPI) using the formula (19): CPI =91− (0.65× % DLco) − (0.53× % FVC) + (0.34× % FEV1).
We then evaluated the results for each clinical, physiological, and radiological variable according to GAP staging.
Acute exacerbation was diagnosed based on Collard criteria (20).
Analysis
The results for continuous variables are given as means ± standard deviations and those for categorical variables are given as percentages. Chi-square and Fisher’s exact tests were used to compare the results for categorical variables, and unpaired t-tests and Mann-Whitney U tests were used to compare the results for continuous variables. Cox regression analysis was used to identify significant factors for predicting patient mortality. Kaplan-Meier survival curves and log-rank tests were used to compare patient survival according to GAP stages. The level of statistical significance was set at P<0.05. STATA software V.11.0 (Stata Corp., College Station, TX, USA) was used for all analyses.
Results
Patient characteristics
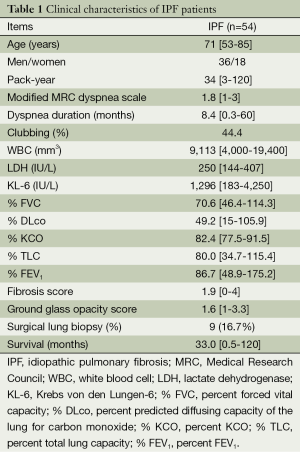
During the study period, we identified 54 IPF patients for whom there were sufficient data. The clinical characteristics of these IPF patients are shown in Table 1. They included 36 men and 18 women, with a mean age of 71 years (range, 53-85 years). Among these patients, 37 were ex or current smokers and had a mean 34 pack-year history (range, 3-120 pack-year). Mean mMRC dyspnea scale scores and dyspnea duration were 1.8 (range, 1-3) and 8.4 months (range, 0.3-60 months), respectively. A total of 24 patients (44.4%) had clubbing. Regarding female patients, we checked connective tissue (CTD) associated symptoms and autoantibodies and ruled out CTD.
Full table
Table 1 shows the laboratory results (WBC counts and LDH, CRP, and KL-6 levels), pulmonary function test results (% FVC, % DLco, % KCO, % TLC, and % FEV1), and mean scores for fibrosis and GGO on chest HRCT. The mean fibrosis score for the lower lung field was 2.5 (range, 1-5). The mean CPI score for these IPF patients was 52.7 (range, 0.6-91). Our interobserver agreement of HRCT scoring data was 0.56.
The overall median survival time of these patients was 33.0 months (range, 0.5-120 months). During this observation period, 32 (59.2%) patients died; of these, 20 (62.5%) died because of acute exacerbations and 6 (18.8%) died from pulmonary hypertension.
IPF clinical characteristics and GAP stages
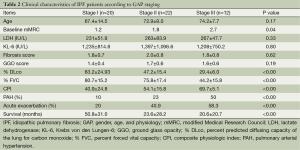
Table 2 shows the clinical characteristics of the 54 IPF patients according to GAP stages I-III. Mean fibrosis and CPI scores according to GAP stages were 1.8 and 40.9 for stage I, 2.0 and 54.1 for stage II, and 1.8 and 69.7 for stage III, respectively. Stage III patients had pulmonary arterial hypertension (PAH) more frequently compared with stage II patients (50% vs. 23%, P<0.036). Mean systolic pulmonary artery pressures of stage III and stage II patients were 52 mmHg (range, 40-66 mmHg) and 40 mmHg (range, 35-48 mmHg), respectively.
Full table
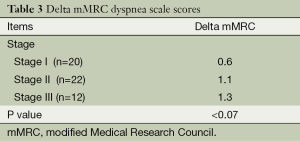
We re-evaluated mMRC dyspnea scale scores at 1 year (Table 3). Within 1 year, 18 patients died. Therefore, we analyzed serial change according to the survival duration. Delta mMRC scores tended to increase with stage III as compared with those with stage I and stage II, although these differences were not significant (P=0.07). Stage III patients tended to have a higher incidence of acute exacerbations than stage II and stage I patients (58.3% vs. 40.9% and 20.0%, respectively).
Full table
With respect to treatment, only 5 patients (9.3%) were followed without any treatment. A total of 27 patients (50%) received prednisolone (PSL) alone, and 17 (31.5%) received both PSL and immunosuppressants. Only 3 patients (5.6%) received pirfenidone alone; these patients belonged to GAP stage I or stage II. These three patients had stable course including FVC and clinical symptoms over 3 years. All stage III patients received medications, including PSL, PSL with immunosuppressants, or PSL with pirfenidone.
Predictors of IPF patient mortality
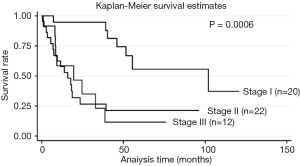
Univariate model showed hazard ratio (HR) of GAP was 1.350 (95% CI: 1.083-1.684; P=0.008). Adjusted for age, we did a Cox proportional hazards model showed that gender (HR: 55.2, 95% CI: 36.31-74.17, P<0.001), % DLco (HR: 1.7, 95% CI: 1.25-2.15, P<0.001), and CPI (HR: 1.8, 95% CI: 1.17-2.51, P<0.001) were strong predictors of mortality (Table 4). HRCT fibrosis score did not predict mortality compared with physiological parameter or gender in our cohort. C-statistics with ROC curve was 0.718. Kaplan-Meier survival curves according to GAP staging indicated that the mean survival time of stages I, II, and III patients was 50.8 months, 23.6 months, and 20.6 months, respectively (P=0.0006) (Figure 1). Between stage II and stage III, stage II showed poor prognosis than stage III at some point.
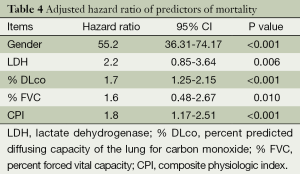
Full table
Discussion
In this study, we determined the clinical characteristics of IPF patients in our hospital on the basis of the simple GAP staging system that was proposed by Ley et al. (14). For physicians, it is important to decide which patient to treat and how to monitor or predict the course of a progressive disease such as IPF. If we can employ simple variables and tools, it may be possible to create an applicable staging system for use in hospitals worldwide. Our study provides crucial findings for each stage of this GAP system for IPF patients.
Stage I patients tended to follow a good clinical course compared with stage II and III patients. This group of IPF patients had no evidence of pulmonary artery hypertension. Based on pulmonary function and CPI values, these patients had better preserved respiratory function. However, Kondoh et al. have shown that some IPF patients develop disease progression without any initial pulmonary function impairment (21). Thus, we should monitor disease activity carefully (22).
Thin-section chest CT scanning is useful for evaluating ILD (23,24). The latest IPF guidelines emphasize the importance of chest HRCT findings (16). Therefore, we selected the fibrosis scoring system devised by Kazerooni et al. to evaluate disease severity because of its easy applicability (15).
With respect to honeycombing, we considered Fleischner Society definitions (18,25). Stage II patients had the highest fibrosis scores and the majority of these patients received aggressive therapy (26,27). In our cohort, stage II patients had good prognosis slightly compared with stage III patients. Stage II patients may have had more good activities of daily living and slow progression partly with intensive treatment. In Figure 1, stage II patients had poor prognosis than stage III at some points. Possible reason is due to progressive fibrosis with high HRCT score. However, HRCT fibrosis score did not predict mortality completely in our cohort. One possible reason is stage II had less often had PAH than stage III. Vasculopathy or hyper coagulopathy may contribute more to both acute exacerbation and mortality in our IPF patients. In terms of physiology and imaging, we should follow up these two stages IPF patients carefully and require comprehensive management.
In contrast, our stage III patients had poor prognosis despite having similar fibrosis scores. One possibility was that the patients in this group had PAH as speculative. For IPF associated with PAH, both monitoring and management strategies have been proposed (28,29). Second is stage III patients had acute exacerbations more frequently compared with the patients in the other two groups. We receive many advanced IPF patients from other local hospital. Therefore, our rather high incidence of AE-IPF may be referral bias. Regarding acute exacerbations in IPF patients, we have recently proposed staging-based management (30).
In addition, our stage III patients exhibited greater increased mMRC breathlessness scale during 1-year observation. From the clinical perspective, cough has been proposed as providing prognostic information for IPF patients (31,32). We believe that this association between changes in mMRC breathlessness scale scores and IPF staging in our cohort is a novel finding. New Idiopathic Interstitial Pneumonias (IIPs) guidelines have proposed considerations of disease behavior (33). Serial monitoring of major clinical symptom trends is crucial for managing this progressive disease.
Finally, with regard to treatment, the antifibrotic drug pirfenidone was effective for stage I and stage II patients in our cohort, similar to the results of previous reports (34-37). However, our stage III patients progressed despite receiving pirfenidone. Recently, patients who showed greater declines in FVC were reported to have demonstrable responses to pirfenidone therapy (38). Sakamoto et al. also reported efficacy of pirfenidone for advanced-stage IPF patients (39). However, there is no consensus regarding which patients should be treated with pirfenidone. We need more large sample size for resolving these controversy issue.
There were several limitations in our study. First, this was retrospective study, small sample size and some clinical data were missing. Second, this was a single-center study with some selection bias. Thus, our results may not be applicable for all IPF patients. However, the epidemiological and clinical characteristics of our cohort were comparable to those of cohorts in previous reports. Third, we evaluated PAH with cardiac echograms and not by cardiac catheterization. Estimated pulmonary arterial pressure by cardiac echo were approximately 10 mmHg higher than catheter evaluation in 40-50%. Severe-stage patients could not undergo such an invasive procedure; thus, it was impossible to perform catheterization for all patients. However, we did comprehensive diagnostic approach including physical examination, blood test and chest imaging. In addition, the clinical courses of our patients with IPF and PAH were consistent with those of similar patients in previous reports.
In conclusion, we determined the clinical characteristics of IPF patients on the basis of GAP staging. Combined evaluations of dyspnea changes, PAH, GAP stages, and fibrosis scores are quite important for disease behavior and for predicting the mortality of IPF patients. Additional multicenter studies are essential for validating our proposal.
Acknowledgements
Author’s contributions: Conception and design: T Kishaba, S Yamashiro. Analysis and interpretation: Y Shimaoka, T Kishaba, H Nagano and Y Nei. Drafting the manuscript for important intellectual content: T Kishaba and Y Nei.
Disclosure: The authors declare no conflict of interest.
References
- Lynch DA, Godwin JD, Safrin S, et al. High-resolution computed tomography in idiopathic pulmonary fibrosis: diagnosis and prognosis. Am J Respir Crit Care Med 2005;172:488-93. [PubMed]
- Nishimura K, Kitaichi M, Izumi T, et al. Usual interstitial pneumonia: histologic correlation with high-resolution CT. Radiology 1992;182:337-42. [PubMed]
- American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 2000;161:646-64. [PubMed]
- Bjoraker JA, Ryu JH, Edwin MK, et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1998;157:199-203. [PubMed]
- Fernández Pérez ER, Daniels CE, Schroeder DR, et al. Incidence, prevalence, and clinical course of idiopathic pulmonary fibrosis: a population-based study. Chest 2010;137:129-37. [PubMed]
- Okamoto T, Ichiyasu H, Ichikado K, et al. Clinical analysis of the acute exacerbation in patients with idiopathic pulmonary fibrosis. Nihon Kokyuki Gakkai Zasshi 2006;44:359-67. [PubMed]
- Martinez FJ, Safrin S, Weycker D, et al. The clinical course of patients with idiopathic pulmonary fibrosis. Ann Intern Med 2005;142:963-7. [PubMed]
- Ley B, Collard HR, King TE Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011;183:431-40. [PubMed]
- Goh NS, Desai SR, Veeraraghavan S, et al. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med 2008;177:1248-54. [PubMed]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Global strategy for the diagnosis, management and prevention of COPD. Available online: www.goldcopd.org, accessed on 2 February 2013.
- King TE Jr, Tooze JA, Schwarz MI, et al. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med 2001;164:1171-81. [PubMed]
- du Bois RM, Weycker D, Albera C, et al. Ascertainment of individual risk of mortality for patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011;184:459-66. [PubMed]
- Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med 2012;156:684-91. [PubMed]
- Kazerooni EA, Martinez FJ, Flint A, et al. Thin-section CT obtained at 10-mm increments versus limited three-level thin-section CT for idiopathic pulmonary fibrosis: correlation with pathologic scoring. AJR Am J Roentgenol 1997;169:977-83. [PubMed]
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [PubMed]
- Bestall JC, Paul EA, Garrod R, et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999;54:581-6. [PubMed]
- Watadani T, Sakai F, Johkoh T, et al. Interobserver variability in the CT assessment of honeycombing in the lungs. Radiology 2013;266:936-44. [PubMed]
- Wells AU, Desai SR, Rubens MB, et al. Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. Am J Respir Crit Care Med 2003;167:962-9. [PubMed]
- Collard HR, Moore BB, Flaherty KR, et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2007;176:636-43. [PubMed]
- Kondoh Y, Taniguchi H, Ogura T, et al. Disease progression in idiopathic pulmonary fibrosis without pulmonary function impairment. Respirology 2013;18:820-6. [PubMed]
- Hanamoto S, Ohsuji T, Tsuyuguchi I, et al. Prediction formulas for pulmonary function tests expressed in linear and exponential form for healthy Japanese adults. Nihon Kyobu Shikkan Gakkai Zasshi 1992;30:2051-60. [PubMed]
- Murata K, Khan A, Herman PG. Pulmonary parenchymal disease: evaluation with high-resolution CT. Radiology 1989;170:629-35. [PubMed]
- Akira M, Sakatani M, Ueda E. Idiopathic pulmonary fibrosis: progression of honeycombing at thin-section CT. Radiology 1993;189:687-91. [PubMed]
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [PubMed]
- Inase N, Sawada M, Ohtani Y, et al. Cyclosporin A followed by the treatment of acute exacerbation of idiopathic pulmonary fibrosis with corticosteroid. Intern Med 2003;42:565-70. [PubMed]
- Kondoh Y, Taniguchi H, Yokoi T, et al. Cyclophosphamide and low-dose prednisolone in idiopathic pulmonary fibrosis and fibrosing nonspecific interstitial pneumonia. Eur Respir J 2005;25:528-33. [PubMed]
- Watanabe N, Taniguchi H, Kondoh Y, et al. Clinical efficacy of sildenafil in interstitial pneumonia with pulmonary hypertension. Nihon Kokyuki Gakkai Zasshi 2011;49:151-5. [PubMed]
- Gläser S, Obst A, Koch B, et al. Pulmonary hypertension in patients with idiopathic pulmonary fibrosis - the predictive value of exercise capacity and gas exchange efficiency. PLoS One 2013;8:e65643. [PubMed]
- Kishaba T, Tamaki H, Shimaoka Y, et al. Staging of acute exacerbation in patients with idiopathic pulmonary fibrosis. Lung 2014;192:141-9. [PubMed]
- Ryerson CJ, Abbritti M, Ley B, et al. Cough predicts prognosis in idiopathic pulmonary fibrosis. Respirology 2011;16:969-75. [PubMed]
- Lechtzin N, Hilliard ME, Horton MR. Validation of the Cough Quality-of-Life Questionnaire in patients with idiopathic pulmonary fibrosis. Chest 2013;143:1745-9. [PubMed]
- Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. [PubMed]
- Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2005;171:1040-7. [PubMed]
- Taniguchi H, Ebina M, Kondoh Y, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J 2010;35:821-9. [PubMed]
- Taniguchi H, Kondoh Y, Ebina M, et al. The clinical significance of 5% change in vital capacity in patients with idiopathic pulmonary fibrosis: extended analysis of the pirfenidone trial. Respir Res 2011;12:93. [PubMed]
- Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 2011;377:1760-9. [PubMed]
- Okuda R, Hagiwara E, Baba T, et al. Safety and efficacy of pirfenidone in idiopathic pulmonary fibrosis in clinical practice. Respir Med 2013;107:1431-7. [PubMed]
- Sakamoto S, Itoh T, Muramatsu Y, et al. Efficacy of pirfenidone in patients with advanced-stage idiopathic pulmonary fibrosis. Intern Med 2013;52:2495-501. [PubMed]