
Evaluation of liquid based cytology in detection of EGFR mutation in NSCLC by large samples
Introduction
The detection of the epidermal growth factor receptor (EGFR) mutation is crucial for the treatment of non-small cell lung cancer (NSCLC) patients. Numerous clinical trials have shown that the progression-free survival (PFS) of NSCLC patients with EGFR mutation and treated with tyrosine kinase inhibitors (TKI) is significantly prolonged (1,2). Approximately two-thirds of NSCLC patients in advanced stages lose the chance of operation, and these patients can only be diagnosed by biopsy or cytology samples (3). In addition to pathological diagnosis, detection of driver oncogene mutation including EGFR target should be conducted. An increasing number of laboratories are applying cytology specimens, including liquid-based cytology specimens, cell blocks, and smears, even plasma to molecular detection, although formalin-fixed and paraffine-embedded (FFPE) specimens remain the primary sample type used (4,5). Liquid-based cytology is convenient, easy to operate, and reliable in molecular testing; however, the positive rate of liquid-based cytology is inconsistent in many reports (6-8). Few reports on the analysis of large samples have been published, and there is also no standard for the procedure of EGFR detection by liquid-based cytology samples, especially for the evaluation of tumor cells before detection. In our previous research, we explored using a standard quality control process of liquid-based cytology specimen testing for EGFR detection (9). Here, to verify the feasibility of using liquid-based cytology to detect the EGFR mutation gene, the EGFR status of 8,029 samples was tested using liquid-based cytology under this standard quality control process. Another 1,934 cases of EGFR mutation were analyzed by using FFPE samples. From this, we attempted to establish a standardized procedure of liquid-based cytology for EGFR molecular detection. We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-2750).
Methods
Case selection
In this study, the EGFR mutation status of 9,963 cases with informed consent were tested, including 8,029 liquid-based cytology samples and 1,934 FFPE samples from September 2015 to December 2019 in Shanghai Pulmonary Hospital. Age, gender, and pathological classification of lung cancer was compared in the 9,963 cases. EGFR mutation features were compared between liquid-based cytology samples and FFPE samples. Pathological diagnosis according to cytology was divided into three subtypes: adenocarcinoma (AC), squamous cell carcinoma (SCC), and NSCLC. Cytology samples included percutaneous fine needle aspiration (FNA), pleural effusion (PE), sputum, bronchial brushing, bronchoalveolar lavage fluid (BALF), and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) samples. FFPE samples included biopsy specimens and surgically resected specimens. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval for the study was obtained from the ethical committee of Shanghai Pulmonary Hospital (approval number: L20-331Y).
Quality control of EGFR mutation detection of liquid based cytology specimens (8)
(I) We evaluate the number of tumor cells in the liquid based cytology slide before EGFR mutation detection. Samples satisfied with more than 50 tumor cells and visible sediment were extracted DNA after centrifugation. If the sample fails to meet this standard, we will suggest the physicians or patients to resample to avoid invalid EGFR detection. (II) We estimated the diameter of precipitation of liquid based cytology specimens. DNA and RNA which can be used to detect anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 receptor tyrosine kinase (ROS1) genes were extracted at the same time, when the diameter was between 2–5 mm. For specimens with precipitation diameter >5 mm, the residual cell pellet was resuspended in the solution and then was divided equally into 2–1.5 mL centrifuge tube. DNA and RNA were extracted separately. The workflow model is listed in Figure 1.
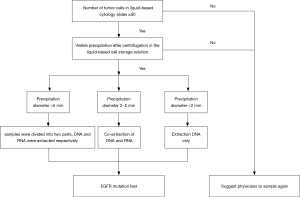
DNA extraction process of liquid based cytology
(I) Liquid based cytological specimens stored in ThinPrep (hologic Gen probe) preservation solution were centrifuged for 12,000 r/min, 3 minutes, and precipitation was transferred to 1.5 mL centrifuge tube. (II) The total DNA was extracted using an AmoyDx DNA Kit (Amoy Diagnostics Co, Xiamen, China) according to the manufacturer’s instructions. The quality and quantity of the DNA was measured on an FLx800 Spectrophotometer (BioTek Instruments, Inc., USA).
DNA extraction process of FFPE samples
(I) For FFPE samples, wax block was cut into 3 rolls (10 µm thick/roll). And then put rolls into 1.5 mL centrifuge tube. (II) Add 1 mL dimethylbenzene into centrifuge tube to dewax (56 °C, 15 min), and then centrifuge for 12,000 r/min, 3 minutes, repeat this step. (III) Discard the supernatant, neutralize the residual dimethylbenzene with anhydrous ethanol. (IV) The total DNA was extracted using an AmoyDx DNA Kit (Amoy Diagnostics Co, Xiamen, China) according to the manufacturer’s instructions. The quality and quantity of the DNA was measured on an FLx800 Spectrophotometer (BioTek Instruments, Inc., USA).
EGFR mutation test
The EGFR gene were detected by ARMS-PCR using an ADx EGFR Gene Detection Kit (Amoy Diagnostics Co, Xiamen, China). The PCR amplification (95 °C for 5 minutes, 15 cycles of 95 °C for 25 seconds, 64 °C for 20 seconds, and 72 °C for 20 seconds; and then, 31 cycles at 93 °C for 25 seconds and 60 °C for 35 seconds) was performed. The status of the genes was detected on a Stratagene Mx3000P QPCR System (Agilent Technologies, Santa Clara, CA, USA).
Statistical analysis
The statistical analyses were performed using SPSS software version 25.0 (IBM, Armonk, NY, USA). Continuous variables were compared using Mann-Whitney U tests. Variables displayed as percentages were compared with a χ2 test. Differences with P<0.05 were considered significant.
Results
Study population and clinical pathological characteristics
In this study, 9,963 cases were enrolled. The age ranged from 19 to 94 years old, and the median age was 65 years old. EGFR mutation were detected in 4,587 cases, the positive rate was 46.04%. EGFR mutation rate was higher in 3,924 female patients (65.07%) than 6,036 male patients (33.68%) (P<0.01). The EGFR mutation rate of AC, NSCLC and SCC was 58.57%, 30.04% and 8.33% accordingly (P<0.01). In our study, 8,029 cases were detected by liquid based cytology specimens, accounting for 80.59%. The EGFR mutation rate of liquid based cytology specimens was 47.18% and FFPE specimens was 41.37%. There was statistical difference between the two specimens (data was shown in Table 1).
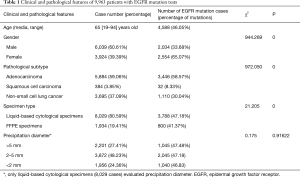
Full table
EGFR mutation rate of different specimens
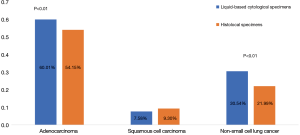
EGFR mutation rate of AC (60.01%) and NSCLC (30.54%) detected by liquid based cytology specimens were higher than FFPE specimens respectively (60.01% vs. 30.54% and 54.15% vs. 21.99%, χ2=13.845, P<0.01 and χ2=16.026, P<0.01). Mutation rate of SCC detected by liquid based cytology specimens was 7.58%, a little lower than FFPE specimens, however, there was no statistical difference between the two groups (Figure 2).
EGFR mutations sites in different types of specimens
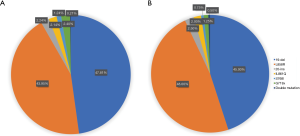
In 8,029 cases detected by liquid based cytology specimens, 1,811 cases were found 19 Del (47.81%) and 1,665 cases (43.95%) were found L858R point mutations. These two EGFR mutation sites were most frequent. Other mutation sites included 85 20-ins cases (2.24%), 81 L861Q cases (2.14%), 47 S768I cases (1.24%), 91 G719X cases (2.4%), and 8 cases (0.21%) which have been found two mutation sites (Figure 3A). In 1,934 cases detected by FFPE specimens, we detected 360 19 Del cases (45.00%), 384 L858R cases (48.00%), 20 20-ins cases (2.50%), 16 L861Q cases (2.00%), 6 S768I cases (0.75%), 10 G719X cases (1.25%), and only 4 double mutation cases (0.50%) (Figure 3B).
EGFR mutations in different sample types of liquid based cytology
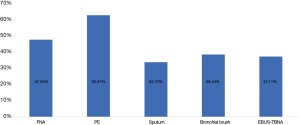
In 6 different sample types, PE has the highest EGFR mutation rate (695/1,109, 62.67%). The second was FNA samples (2,372/4,988, 47.55%). The EGFR mutation rate of bronchial brush and EBUS-TBNA was 38.44% (316/822) and 37.11% (380/1,024). The lowest EGFR mutation rate was sputum (29/86, 33.72%) (χ2=180.122, P<0.01). Data was shown in Figure 4.
Efficacy of TKI therapy in patients with EGFR mutations
Among 3,788 patients with EGFR mutation tested by liquid-based cytological specimens, we followed 107 cases to track the efficacy of patients with positive EGFR testing. Fifty-seven patients (53.27%) achieved partial response (PR) within 1 month, 41 patients (38.32%) achieved stable disease (SD), only 9 patients (8.41%) showed disease progression (PD) in 1 month. The objective response rate (ORR) was 53.27% and disease control rate (DCR) was 91.59%.
Discussion
It’s very important for NSCLC patients to detect drive mutation, as it’s essential for therapeutic regime and prognosis. In various types of drive mutation, EGFR gene mutation is the most frequent one (10). Accurately screened out EGFR mutations in NSCLC can significantly improve the prognosis of patients. To NSCLC patients without indication of surgery, cytology specimen is the only pathological sample available. Cytology samples can not only provide pathological diagnosis, but also can be used to detect gene mutation in NSCLC patients (11). In addition to liquid based specimens, cell block embedded specimens and cell smear scraping specimens are also used for molecular detection (12,13). However, it is difficult to make a cell block embedded specimen successfully in case of little cells, and cell smear, sometimes as the only pathological slide of patients, will not be filed after scraping. Liquid based cytology is the preferred specimen for molecular detection in Cleveland Clinic in the United States, according to Doxtader et al. (14). Therefore, the use of liquid based cytology is particularly important for the detection of gene mutation. In our previous study, we established an evaluation system of EGFR mutation detection by liquid based cytology specimens. Here, we summarized our previous experience regarding EGFR gene mutation based on 8,029 cases of cytology samples and evaluated the mutation frequency of EGFR in NSCLC patients in China.
The EGFR mutation rate of NSCLC patients tested by liquid based cytology samples was quite different in literatures (3,4). There are many associated factors. The most important one is related to the pre-treatment of specimens and the establishment of evaluation before EGFR mutation test. A large number of samples were explored in our laboratory in the early stage, and the evaluation of tumor cell content of liquid-based cytology samples before molecular detection was established, and it was applied to daily work. For the cases with less than 50 tumor cells in the liquid based cytology slide, it is not recommended to use this liquid based cytology sample for EGFR mutation detection. Even if the diameter of centrifugation precipitation is large, there are possibility to have plenty of normal epithelial cell or other components mixed. In this case, the tumor cells account for less, when the EGFR mutation test result is negative, it may be a false negative. It is recommended to sample again or use more sensitive detection methods, such as super arms PCR, to detect molecular mutation. It is also not suitable to test the samples with too small sedimentation diameter which was invisible to the naked eye, because the results also have the possibility of false negative, and also the high cost of gene test brings the patients high economic burden. If there is no other samples obtained or no chance to resample again, EGFR mutation test can be done by this unqualified sample after fully communication with patients and clinicians, however, it is not recommended to carry out test in daily work.
In our study, the EGFR mutation rate in women was significantly higher than that in men. And the EGFR mutation rate was highest in AC and lowest in SCC, which was consistent with the literature (15). Further analysis showed that the EGFR mutation rate in AC and NSCLC tested by liquid based cytology samples was higher than FFPE samples. It has been reported that cytology samples can provide high quality DNA for molecular detection, and the results are consistent with those of FFPE samples including surgical resection samples and biopsy samples (16). Because of the high proportion of tumor cells in cytology specimens, cytology specimens were more suitable for molecular detection according to Roy-Chowdhuri et al. (17). On the other hand, for liquid based cytology specimens, pathological diagnosis only depends on cell morphology. Poor differentiation NSCLC lacks the typical cell morphology of AC and SCC, and cannot be further defined by immunohistochemistry, resulting in a proportion of poorly differentiated AC, SCC, adenosquamous cell carcinoma and large cell carcinoma in NSCLC group of cytological specimens. Therefore, the EGFR mutation rate was higher in NSCLC group. We believe that EGFR should be tested in NSCLC patients, especially those who depend on cytological diagnosis, so as to avoid missing the opportunity of EGFR TKI treatment.
According to the different sampling methods, the liquid based cytology samples are divided into FNA, PE, sputum, bronchial brush, BALF and EBUS-TBNA samples. Among these samples, we found PE had the highest EGFR mutation rate, reaching 63.35%. There are two reasons for the high EGFR mutation rate in PE. First, AC is the most common pathological type of malignant pleural effusion in lung cancer patients (18). Secondly, a series of recent reports showed that lung cancer has large heterogeneity in tumor (19). For example, the gene characteristics of primary and metastatic lesions will also be different, and the DNA of tumor cells can also be released into PE through necrosis or apoptosis of tumor cells (20). Therefore, PE can detect the presence of gene mutation better than a biopsy sample with limited tumor cells (20). In recent years, many studies have also shown that the detection of driving mutation in PE can provide reliable molecular detection results for patients treated with TKI, and even the supernatant of pleural effusion with negative cytological diagnosis can also be used as reliable liquid biopsy samples (21,22). PE can be collected quickly, minimally invasive and repeatedly. For patients with advanced NSCLC patients with PE, it is recommended to send PE samples for EGFR mutation detection and EGFR TKI treatment monitoring.
For NSCLC patients with EGFR-exon 19 deletions or an exon 21 Leu858Arg mutation, EGFR TKIs can improve response rates, time to progression, and overall survival. Consist with literature (23), we found the ORR was 53.27% and disease control rate (DCR) was 91.59%, only 8.41% patients showed PD in 1month. There are different mechanisms of acquired resistance to EGFR TKIs. T790M mutation, HER2 amplification, MET amplification and small cell lung cancer transformation are among the highest (23).
Since next-generation sequencing hasn’t carried out universally, we haven’t compared the results of EGFR gene mutation detection with it. In future study, EGFR gene status will be compared between ARMs and next-generation sequencing.
In conclusion, EGFR detection with liquid-based cytological sample is less invasive and easy to obtain samples. When establishing standardized process and evaluation system of samples, it can provide accurate detection results to guide TKI treatment, and it can also monitor drug resistance of NSCLC patients after TKI treatment. It is recommended to promote its application. In addition, EGFR mutation detection is still recommended for patients with NSCLC, especially diagnosed with cytology samples. In all kinds of cytology specimens for the EGFR detection, PE is first choice.
Acknowledgments
Funding: This research was supported by Shanghai Municipal Commission of Heath and Family Planning (NO. 201740134).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-2750
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-2750
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval for the study was obtained from the ethical committee of Shanghai Pulmonary Hospital (approval number: L20-331Y), and written informed consent was obtained from the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See:
References
- Okamoto I, Takahashi T, Okamoto H, et al. Single-agent gefitinib with concurrent radiotherapy for locally advanced non-small cell lung cancer harboring mutations of the epidermal growth factor receptor. Lung Cancer 2011;72:199-204. [Crossref] [PubMed]
- Uruga H, Kishi K, Fujii T, et al. Efficacy of gefitinib for elderly patients with advanced non-small cell lung cancer harboring epidermal growth factor receptor gene mutations: a retrospective analysis. Intern Med 2010;49:103-7. [Crossref] [PubMed]
- Walker S. Updates in non-small cell lung cancer. Clin J Oncol Nurs 2008;12:587-96. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:504-35. [Crossref] [PubMed]
- Arriola E, Paredes-Lario A, Garcia-Gomez RJ, et al. Comparison of plasma ctDNA and tissue/cytology-based techniques for the detection of EGFR mutation status in advanced NSCLC: Spanish data subset from ASSESS. Clin Transl Oncol 2018;20:1261-7. [Crossref] [PubMed]
- Wu CY, Hou LK, Ren SX, et al. High feasibility of liquid-based cytological samples for detection of EGFR mutations in Chinese patients with NSCLC. Asian Pac J Cancer Prev 2014;15:7885-9. [Crossref] [PubMed]
- Zhao H, Qiu T, Guo H, et al. Detection of EGFR and KRAS gene mutations using suspension liquid-based cytology specimens in metastatic lung adenocarcinoma. Oncotarget 2017;8:106685-92. [Crossref] [PubMed]
- Satoh Y, Matsuo Y, Kuba T, et al. EGFR mutation genotyping and ALK status determination in liquid-based cytology samples of non-small cell lung cancer. Virchows Arch 2020;476:753-62. [Crossref] [PubMed]
- Passaro A, Guerini-Rocco E, Pochesci A, et al. Targeting EGFR T790M mutation in NSCLC: From biology to evaluation and treatment. Pharmacol Res 2017;117:406-15. [Crossref] [PubMed]
- Han B, Tjulandin S, Hagiwara K, et al. EGFR mutation prevalence in Asia-Pacific and Russian patients with advanced NSCLC of adenocarcinoma and non-adenocarcinoma histology: The IGNITE study. Lung Cancer 2017;113:37-44. [Crossref] [PubMed]
- Wu S, Shih J. Management of acquired resistance to EGFR TKI_targeted therapy in advanced non-small cell lung cancer. Mol Cancer 2018;17:38. [Crossref] [PubMed]
- Dong Z, Li H, Zhou J, et al. The value of cell block based on fine needle aspiration for lung cancer diagnosis. J Thorac Dis 2017;9:2375-82. [Crossref] [PubMed]
- Magnini D, Fuso L, Varone F, et al. Molecular Testing in EBUS-TBNA Specimens of Lung Adenocarcinoma: A Study of Concordance Between Cell Block Method and Liquid-Based Cytology in Appraising Sample Cellularity and EGFR Mutations. Mol Diagn Ther 2018;22:723-8. [Crossref] [PubMed]
- Doxtader EE, Cheng YW, Zhang Y. Molecular Testing of Non-Small Cell Lung Carcinoma Diagnosed by Endobronchial Ultrasound-Guided Transbronchial Fine-Needle Aspiration: The Cleveland Clinic Experience. Arch Pathol Lab Med 2019;143:670-6. [Crossref] [PubMed]
- Hsieh RK, Lim KH, Kuo HT, et al. Female sex and bronchioloalveolar pathologic subtype predict EGFR mutations in non-small cell lung cancer. Chest 2005;128:317-21. [Crossref] [PubMed]
- Gailey MP, Stence AA, Jensen CS, et al. Multiplatform comparison of molecular oncology tests performed on cytology specimens and formalin-fixed, paraffin-embedded tissue. Cancer Cytopathol 2015;123:30-9. [Crossref] [PubMed]
- Roy-Chowdhuri S, Chen H, Singh RR, et al. Concurrent fine needle aspirations and core needle biopsies: a comparative study of substrates for next-generation sequencing in solid organ malignancies. Mod Pathol 2017;30:499-508. [Crossref] [PubMed]
- Akamatsu H, Koh Y, Kenmotsu H, et al. Multiplexed molecular profiling of lung cancer using pleural effusion. J Thorac Oncol 2014;9:1048-52. [Crossref] [PubMed]
- Zhang J, Fujimoto J, Zhang J, et al. Intratumor heterogeneity in localized lung adenocarcinomas delineated by multiregion sequencing. Science 2014;346:256-9. [Crossref] [PubMed]
- Han HS, Eom DW, Kim JH, et al. EGFR mutation status in primary lung adenocarcinomas and corresponding metastatic lesions: discordance in pleural metastases. Clin Lung Cancer 2011;12:380-6. [Crossref] [PubMed]
- Pang C, Ma H, Qin J, et al. Pleural effusion as a substitute for tumor tissue in detecting EGFR/ALK mutations in non-small cell lung cancer: A systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e15450. [Crossref] [PubMed]
- Song Z, Wang W, Li M, et al. Cytological-negative pleural effusion can be an alternative liquid biopsy media for detection of EGFR mutation in NSCLC patients. Lung Cancer 2019;136:23-9. [Crossref] [PubMed]
- Wang S, Chen H, Zhong J, et al. Comparative study of EGFR mutations detected in malignant pleural effusion, plasma and tumor tissue in patients with adenocarcinoma of the lung. Lung Cancer 2019;135:116-22. [Crossref] [PubMed]
(English Language Editor: J. Gray)


