The global COVID-19 pandemic at a crossroads: relevant countermeasures and ways ahead
Introduction
Since the outbreak of novel coronavirus disease (COVID-19) in Wuhan, China at the beginning of December 2019, over 83,500 cumulative infected cases and 4,634 death cases have been confirmed in mainland China as of 4th July, 2020. There were over 11 million confirmed cases outside China, affecting 213 countries in the world and two international conveyances with nearly 530,000 deaths. The pandemic has been sweeping all continents, and North America, Latin America Europe, Russia, Middle East, South Asia among others at an alarming rapidity (1). The World Health Organization (WHO) raised the world risk level of the COVID-19 pandemic to “very high” on the 28th February, 2020. Hundreds of millions of people would likely be exposed to the virulent epidemic should the current transmission continue rampaging through the global community (2). Currently, the situation is particularly severe in the United States, Brazil, Russia, India, Spain and Peru and Italy, each of whom has already over 300,000 confirmed cases. The United States posted a record 47,000 new COVID-19 cases on 30th June 2020, the biggest one day increase in new infections since the start of the pandemic. In view of sweeping move of the virus, many countries already implemented lockdown policy with draconian travel restrictions, such as the United States and Spain took the toughest measures implemented outside of mainland China to get the COVID-19 pandemic under control. A brief review of the development of the pandemic and the attitude and measures of national government in dealing with the virus prevention and control can help us understand the role of transmission network in order to draw useful lessons for effective control as containment is still apt (3,4).
The coronavirus has the highly contagious nature, which can be spread via person-to-person channel among close contacts between local residents & travellers. Besides, ineffective preventive measures were taken during early stage of the pandemic outbreak by the local authority together with the Chinese Spring Festival transportation (5). The pandemic spread was particularly fast during the period of late January and early February, 2020, with the number of the confirmed infections increasing dramatically in mainland China, from 830 cases (Wuhan: 495; Hubei: 549) on the 23rd January, 2020 to 72,436 cases (Wuhan: 42,752; Hubei: 59,989) on the 17th February, 2020.
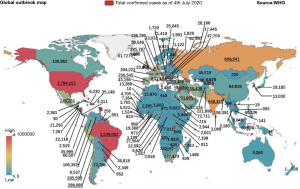
Since the middle of January, the 2019 novel coronavirus (2019-nCoV) was no longer contained in China and new cases have been increasingly exported internationally (6). Outside China, a number of countries around the world successively reported infected cases, which grew rapidly in the middle of February 2020. As of 3rd July, 2020, the number of the cumulative confirmed cases of COVID-19 around the world exceeded the threshold of 11,000,000 people (7). The current distribution of COVID-19 pandemic cases around the world is shown in Figure 1 (7).
This novel respiratory virus has so far spread to more than 210 countries across the world, and a pandemic may be on the way should no effectively control measures were to be implemented at the global level immediately (4,8). While the number of new infection cases in mainland China has been declining thanks to the strict quarantine measures (i.e., including lockdown of the city of Wuhan and provinces, suspension of public transport and all outdoor activities, construction of makeshift hospitals to confine infected persons) put in place by the Chinese authority and no new case has been reported outside of Hubei province since the end of February, the situation in some other countries such as the United States, Russia, Brazil, Spain, United Kingdom, Italy, started deteriorating rapidly, and it was rapidly developed into a global pandemic at the end of March due to lack of global coordination in effective epidemic containment policy as well as delayed implementation of efficient control measures by the subsequently infected countries.
As of 4th July, 2020, 54,442 new cases were confirmed and the cumulative number of confirmed cases reached 2.8 million in the United States. There were 42,223 new cases and the cumulative number of confirmed cases reached 1,539,081 in Japan. In Russia, the number of confirmed cases rose to 667,883. In India, 22,771 new cases were reported and the cumulative number of the confirmed cases reached 648,315 according to the Indian Ministry of Health and Family Welfare. There were 442 new cases and the cumulative number of confirmed cases reached 250,545 in Spain [429 death (7)]. A recent research conducted by John Hopkins University shows that the mortality rate in Europe (especially in San Marino, Belgium, Andorra, United Kingdom and Spain)1 has been the highest so far outside China and the underlying reason remains unknown.
Source: Figure 1 is drawn by the authors with the data issued from WHO.
A central and urgent question pertaining to the global pandemic research community and health policymakers is what type of policy measures require to be adopted at the early stage of pandemic outbreak in order to effectively constrain the spread of virus at both local and global levels in the event of a second wave of growing infections. The pandemic control experiences gained in China may be informative to health decision-makers elsewhere in the newly infected regions and countries (9,10). Two different attitudes of the policy responses have been identified among the infected countries since the COVID-19 outbreak, which may be categorised by interventionist versus laisser-faire. The former is concerned with active interventions including radical confinement and quarantine measures, whereas the latter takes rather a sangfroid position in dealing with the virus transmission without active intervention measures. Italy, as the most seriously affected country by the pandemic in Europe, has taken strict prevention measures similar to the Chinese pattern, i.e., close down the seriously infected towns and regions in order to make the interpersonal spread under control since the early stage.
In the late February 2020, an outbreak occurred in Iran, and the Iranian government took a series of intervention measures, e.g., closed down public places, cancelled sports events, and suspended school nationwide. At the same time, the Iranian public strictly abide by the pandemic prevention measures issued by the government. By contrast, countries such as South Korea and Singapore only provide medical advice to the general public about the prevention practices without taking compulsory measures, notwithstanding blocking the severely affected areas which allowed the pandemic enter the community transmission stage. In the meantime, the Japanese government also did not restrict any public activities nor take preventive measures during the early stage of transmission in the country. An outbreak in Japan with similar scale in South Korea could jeopardise international events in Asia such as the scheduled Tokyo Olympic Games this summer. Likewise, the French health authority initially advised the general public that there was no need to shut down schools and public places and normal activities may be maintained without specific quarantine measures except for cancelling mass gatherings involving more than 5,000 people (11). However, a few weeks a later, the French authority realised the urgency of the outbreak and finally imposed the lockdown policy and quarantine measures on 16 March2.
In view of the controversial debates around the proper measures and diverging attitudes of government intervention between different countries, it is necessary to understand that the consequence of different policy measures within a robust analytical framework is of critical importance for the well-being of world population as these measures will lead the development of highly contagious pandemic to various directions. Given the limitation and scarcity of global medical resources, the public health efforts in each country or region should be focused on a few key factors influencing the COVID-19 transmission routes and infection rates, and relevant strategies and measures should be adopted aiming at maximising the effectiveness of policy intervention. Thus, it is necessary and urgent for policy makers to know which prevention and control measures are more likely to succeed in constraining the spread of infections at a reasonably acceptable social cost. A number of prior studies have addressed this issue around prevention effectiveness by using different modelling paradigms (1,12), many of which employed conventional bio-mathematical modelling strategies to describe the dynamics of the spread of infectious diseases such as Susceptible-Exposed-Infectious-Removed (SEIR) model (5), or stochastic transmission model (6). However, few of them has taken the network effect into the modelling consideration.
As mentioned earlier, the person-to-person spread was confirmed by medical experts as the primary infection channel at the early stage of COVID-19 outbreak in Wuhan, although the global epidemiology and infection research have reached a consensus that timely intervention at the initial stage of pandemic is crucial for constraining the spread of infection. The question as to which type of prevention measures is the most effective and what medical intervention resources should be coordinated and mobilised remains inconclusive among the pandemic control community vis-à-vis the ever-increasing new cases around the world, and there is still no signs of whether we are about to reach a global plateau of contamination in the next few weeks, even months under the current tread.
To bridge this knowledge gap, this paper develops a Cellular Automata (CA) configured SEIR model to estimate the transmission properties of COVID-19 by considering the determining factors and the key parameters which are tuned based on the information about infection and transmission under different scenarios. A special advantage of embedding CA into SEIR model is that it allows the pandemic model to take the probabilistic network characteristics into consideration to better reflect the random behaviour of interpersonal virus transmission instead of using fixed parametric estimation. Our research in the prior literature on pandemic modelling indicates that this sophisticated hybrid approach has not been explored in the previous studies. By varying the isolation rate, the contact number during gathering and at regularity, the size of the initial infected cases, and the number of imported cases, we show how to recommend effective containment strategy to the countries at high risk of domestic and imported cases by using contact tracing or isolation measures.
Based on the pandemic development and prevention & control measures in China, similarity of the geographic transmission pattern, medical treatment importance and air travel volumes, the results presented in this study would have implications for proactive prevention measures and medical resource deployment in countries and regions under high risk of COVID-19 pandemic infections, especially in East Asia and Western Europe, as well as religious gathering areas such as Middle East.
We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-1315).
Methods
We implement a dynamic model, in which the CA configured via the SEIR pandemic model is used to simulate the transmission of the COVID-19 novel coronavirus (13). Different from general dynamic models, CA is not determined by strictly defined physical equations or functions, but it is composed of rules constructed by a series of models. CA is a methodological framework where the time, space and state are discrete, each variable only taking a limited number of states, and the rules of state change are local in time and space. The CA was originally developed to simulate the self-replication phenomenon of life system which shares a great similarity with the coronavirus transmission impacted by many stochastic factors (14), which can be used for infectious disease analysis with CA-SIR modelling (15). A based SEIR-IBM (individual based model) model was developed to study the density of population (16). Besides, CA can be used to simulate the virus in the human body and T cell dynamic transformation process after being infected by HIV (17). Through adjusting the parameters of the model, the influence of different factors on the spread of virus can be analysed, and the virus spread pattern under varied parameters setting can be traced so as to analyse the effectiveness of the prevention and control strategy.
Topology of human contact network
It is well known that human contact is a complex network, and its spatial abstract structure can be illustrated in Figure 2. It must be noted that the actual human contact network does not strictly follow the power law distribution, but conform to the local priority connection mechanism. In this paper, a network growth model based on SEIR and CA is proposed to simulate the virus propagation. The procedure of modelling network growth is summarised as follows.
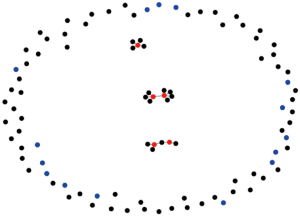
We initialized a random network with probability P0. Considering the mobility of people, human contact characteristics and the time consumption during flow, one or several new nodes are added to the network at each time interval, where each new added node can randomly connect to Node A (i.e., the defined Node) and its neighbours with certain probability.
Virus propagation model structure
In order to accurately represent the COVID-19 pandemic spread, an improved SEIR model with CA (CA-SEIR) is introduced to model the virus propagation, while the state evolution of the model is illustrated in Figure 3. In this model, three primary virus control measures are taken into account: (I) a 14-day quarantine for an individual who is susceptible to be contaminated after exposing to the infected; (II) individuals are not allowed to participate in any gathering and outdoor public activities are closed; (III) construction of makeshift hospitals to admit the patients with mild infection.
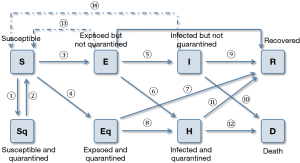
In Figure 3, S, E, I and R refer to the number of susceptible, latent, infectious, and recovered individuals (18); Sq, Eq, H and D are the quarantined susceptible, quarantined latent, quarantined infectious and dead individuals. Suppose the number of each individual contacting with other individuals in the unit time (one day) follow the Poisson distribution, the probability of individual k contacting with individual j in one day can be estimated by,
[1]
where Dek is the node degree of individual k in the interpersonal contact network; λ is the average number of each individual contact with other people in one day. The probability of the individual i being infected by the virus can be calculated by,
[2]
where n is the total number of individual nodes, and the infection probability pik is
[3]
where lik is the infection probability when individual i contacting with individual k; Ik is the infection intensity corresponding to the state of individual k. The form of pik is similar to the PR in PageRank algorithm (19), where individual k assigns its infection effect to all adjacent nodes including individual i.
The individuals are in the latent after virus infection and they can be divided into isolated and non-isolated states considering the different importance degree paid by countries and regions (20). The non-isolated latent individuals with larger node degree are easier to be infected and larger threat to the other individuals in interpersonal contact network. Although small portion of the individuals are immune to the virus, the mutation of the virus would turn them into susceptible state and the virus would evolve with the above pattern in the contact network (21).
CA-SEIR model is a new virus propagation model, in which the CA model treats each individual in the population as a cell and each cell has a different state varying along with the time. SEIR model designs the state changing rules based on the characteristics of the actual epidemiology. By combining CA and SEIR models, the cellular set can be changed according to the characteristics of virus transmission, so as to simulate the transmission process of virus in the population over time.
The parameter settings of the CA-SEIR model is shown in Table 1, where q1 is the isolation rate of the exposed cases; q2 is the isolation rate of the infected cases. “Normal contact times” is the average contact number of each individual under no assembly condition. “Gathering content times” is the average contact number of each individual under assembly condition. “Initial infected number” is the infected cases under no intervention condition. “Medical level” is the index of medical condition (set value based on the released public data, as 0.0413) and “Number of imported” is the number of the imported infected cases (22).
Full table
One advantage of CA-SEIR model is that it takes into account the population status under the isolation condition and the comprehensive influence of various influencing factors, which is more in accordance with the actual situation. The traditional SEIR models estimate the transmission by solving the differential equation constructed with the fixed designed parameters, which does not conform to the characteristics of the randomness of the virus transmission process. Therefore, the CA-SEIR model can simulate the change of population such dynamic process by iterations while the dynamically updated parameters can be used to describe the gathering activities, imported cases, medical conditions in the actual virus transmission situations.
Results
Isolation rate
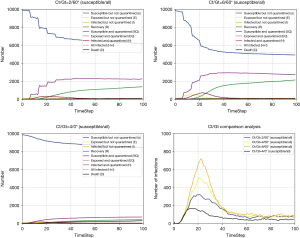
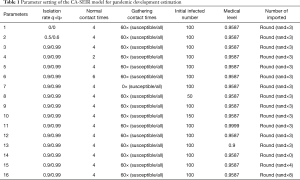
Isolation can effectively block the virus transmission and protect the susceptible individuals (23,24). We implement CA-SEIR model to simulate the pandemic development trend with different isolation rates corresponding to the latent cases and confirmed cases (see Figure 4). The higher the isolation ratio, the lower the total number of infected individuals of the COVID-19. The subgraph of Figure 4 shows that with an isolation rate of 0, the other parameters still influence the virus spread. For instance, on the 23rd January, 2020, the city of Wuhan shut down its public transport. A week later, traffic restrictions were applied throughout China, which greatly increased the isolation ratio. After a 10-day buffer period, the number of newly diagnosed cases in China becomes stable and then gradually declined, which proved that the enhanced quarantine level exerted a positive effect on controlling the spread of the pandemic after the outbreak.
Contact number
A large number of susceptible people could be infected by the virus carriers when they participate in mass gatherings such as the case of large number of infections between Church worshipers in the city of Daegu in South Korea (25). The prevention and control of the gathering in response to pandemic spread is an important but difficult to implement in many parts of the world at present. To take this network effect into account, we use the CA-SEIR model to simulate the development trend under the conditions of different contacts number (Figure 5). The number of potential and confirmed cases would be reduced as the number of contacts decreased. Therefore, the “mobile source of infection” can greatly increase the risk of susceptible people being infected in an intensive social network characterised by large number of interconnections and nodes (a typical example is the megacity with over tens of million people living and interacting closely to each other), leading to the rapid spread of the pandemic. In the early stage of the outbreak, South Korea strictly prevented the importation of the pandemic, whereas the domestic mobility and interpersonal contacts were not regulated in already-infected areas, and a large number of people still participated in the religious gatherings such as the organised masses of the congregants of the Shincheonji Church of Jesus without effective preventive measures put in place. The outbreak began in South Korea on February 18 after a church believer was diagnosed to be infected with COVID-19, with more than 6,000 new cases confirmed in the next two weeks, making South Korea a country with the largest number of infections outside of China. Compared with the stable situation of the early outbreak in South Korea and the consistent growth of the pandemic in China, the explosive growth of the pandemic in South Korea over the past weeks reflects that the population gatherings can greatly increase the spread rate of the pandemic. In order to curtail the fast contamination rate in countries like South Korea, strict quarantine measures with effective isolation decision in the most affected areas have to be made without delay to sharply reduce interpersonal contact, otherwise the entire situation may evolve easily into an irreversible pandemic dilemma.
The number of infections before the intervention
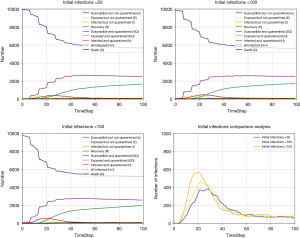
The number of infection cases before intervention depends largely on how quickly the reaction and intervention made by the countries in the initial stage of the outbreak (26). We simulate the development trend of pandemic under different initial infected cases. The more the number of people infected before the intervention, the faster the rate of early cases increased (see Figure 6).
Comparing the pandemic data of South Korea and Germany, we found that South Korea had more than 200 confirmed patients when it took measures such as strict entry control on 21st February, 2020, and the domestic pandemic data has increased rapidly since then. In contrast, Germany adopted strict immigration control measures when about 40 patients were confirmed on 27th February, 2020, and the growth of pandemic data in Germany was relatively slow for a period of time. Thus, the number of people infected before the pandemic prevention measures taken may determine the initial spread rate of the pandemic, leading to markedly different outcome of infections.
Medical level index
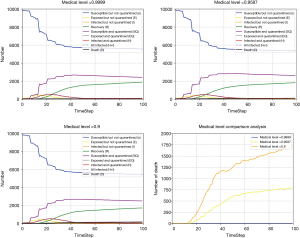
The number of medical personnel involved in the treatment, the length of case diagnosis and the number of isolation beds with negative pressure such medical condition level, are directly related to whether the suspected cases can be diagnosed and treated timely and effectively (27). We evaluate the influence of medical level on pandemic development with CA-SEIR model under different medical levels (Figure 7). With higher medical level index, the death rate of the patients is lower.
At the onset of COVID-19, the medical resources in Hubei province were severely insufficient, and the medical conditions could not cope with the outbreak, so the mortality rate was relatively high. The Chinese government rushed to build special hospitals which began receiving patients on the 8th February, 2020, and the national medical forces were gathered in Hubei province, so that the medical resources of Hubei province were replenished and the treatment of patients was more adequate. After 8th February, 2020, the number of new deaths per day showed a slowing increase trend and gradually declined, which fully reflected the impact of medical conditions on the pandemic mortality rate.
Number of imported cases
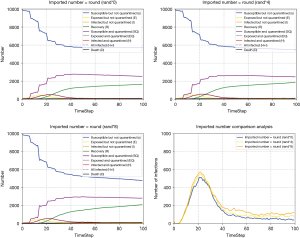
China has gradually changed from an exporting country to the importing country. Without effective control, the importation cases may lead to another outbreak of the pandemic, making it more difficult for China to control the pandemic (28). We also simulated the influence of imported cases on pandemic development with CA-SEIR model (Figure 8). The more imported cases, the more difficult to effectively control the pandemic. In Figure 8C, we can see that when the number of imported cases reaches a certain level, the number of infected cases is likely to cause secondary growth.
On the 1st February, 2020, the first case of COVID-19 in Japan was discovered while the Diamond Princess was docked in Okinawa. On the 3rd of February, 2020, the Japanese government announced to quarantine the entire ship, requiring all passengers to remain on board for 14 days. As of 20th February, 3,711 passengers and crew of the Diamond Princess had confirmed 634 cases of COVID-19. On the same day, the second group of 500 passengers began to disembark and take buses to Yokohama station and other places. Since then, the number of confirmed cases of COVID-19 in Japan has increased rapidly. Therefore, it is extremely important to control the imported cases for preventing and control the pandemic.
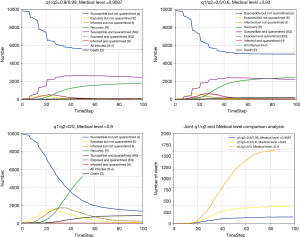
Joint isolation rate and medical level variation
Here, the isolation rate and medical level varied simultaneously are considered, where it is assumed that the medical level is positively correlated with the isolation rate. Therefore, three pairs of the parameters are set and the results demonstrates that the number of the death under zero isolation and poor medical condition is larger than the other two cases. Furthermore, compared the results of Figures 7,9, it can be seen that the isolation rate plays more vital role in the pandemic development.
In summary, a primary lesson of the CA-SEIR model suggests that the proactive measures such as prevention and control are more important than the medical countermeasures including diagnostics and clinical treatment for containing the spread of COVID-19 due to the fact that specific pharmaceutical treatments are not available and the vaccines are still under development (22).
The parameter adjustment simulation clearly shows that restrained interpersonal contact and social interactions in a given pandemic outbreak zone is the most effective strategy to minimise the spread of virus. The significance of analysing the determining factors of the transmission of the COVID-19 is to control and quarantine the infection sources, reduce the transmission route, protect the susceptible population so as to constrain the threat of health posed by the COVID-19 (23).
Discussion
Our hybrid modelling results clearly indicate the paramount importance of taking timely preventive measures in public health system when dealing such contagious pandemic as COVID-19 given its high person-to-person transmission risk, as disastrous consequences would be generated if the optimal window of intervention were forfeited at the early stage of outbreak, in particular in the densely populated areas (i.e., the intra-network transmission probability may be increased exponentially) with scarce medical resources. The prevention and control measures of infectious diseases mainly include strengthening proactive pandemic management and implementing integrated public health intervention measures (29). In the aspect of proactive pandemic management, emergency response should be launched in time supported by early warning system response and a prirori major risk assessment. During the initial prevention period, quantitative analysis can be carried out with a CA-SEIR type model proposed in this study by focusing on such key control parameters as the estimated transmission rate, evaluating the possible impact of the pandemic according to the preliminary epidemiological investigation, and predicting the required resources, scale and time, etc., to formulate and implement the intervention plan aided by medical experts.
Validation of model: case study of Wuhan
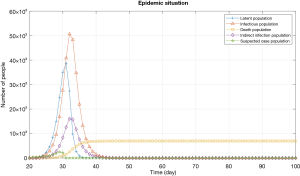
Here we use the COVID-19 outbreak situation in Wuhan as a real case to validate our previous CA-SEIR model simulations with varying determining parameters. The involved prevention and control measures taken in Wuhan and China are listed in Table 2. Figure 10 is the simulated results under the combination of all prevention measures by an improved SEIR model. The model predicted that the number of the cumulated infected cases in Wuhan would reach the highest level (around 50,000) approximately day-40 from the starting of lockdown on the 23rd January, 2020. The actual growth rate of the confirmed cases (ratio of number of infections to the total population) in Wuhan systematically remained below 0.843% since the 23rd January, which demonstrating that the pandemic situation within the city’s boundary has been under control, which is consistent with the actual data of the pandemic situation. The total infected cases in Wuhan were 49,991 on March 2020, and daily new cases was only two-digit since early March, indicating that our modelling results are consistent with the actual data, and it is reasonable to say that inflection point has already passed during the writing of this manuscript. Moreover, the Chinese experience in dealing with COVID-19 can be exported to the other countries facing the emerging risks of mass infections caused by uninterrupted interpersonal transmissions, which can make effective contributions to the global containment (2,3).

Full table
Policy implications
The main purpose of preventive intervention is to eliminate or minimise the source of infections, to cut off the route of transmission and to protect the susceptible population, i.e., reducing public gatherings and close interpersonal contact, good hygiene practice such as wearing masks outdoors and washing hands frequently, cancelling entertainment, social and religious gathering activities, limiting traffic and mobility, strengthening quarantine at transportation hubs such airports, railway and bus stations, closing temporarily schools and public places, and carrying out thorough disinfection when necessary (25). In addition, to increase the efficiency of isolation of infected population, early detection, diagnosis and treatment are highly recommended, including routine temperature detection of vulnerable population, screening and monitoring of fever patients, centralised isolation of the suspected cases and confirmed patients. In the event of high risk of contamination, more draconian quarantine measures such as home confinement of the entire infected areas with tight mobility restriction should be implemented (such as the case of lockdown in Wuhan and North Italy) (30).
In order to optimize the efficacy of pandemic prevention and control measures, implementation guidelines need to be formulated for effective infectious disease control, by clearly defining the responsibilities of medical institutions and public health institutions at all levels, while coordinating personnel from health care, public security, transportation and social service departments of local community. An integrated resources management and allocation system needs to be established to allow local medical and social workers to jointly complete the work of screening and controlling the sources of infection. The success of joint implementation of the pandemic control guideline depends upon the response of emergency plan such as cutting off the route of transmission and protecting the susceptible population with isolation and treatment of infectious diseases (31).
With the rapid spread of novel coronavirus worldwide, this pandemic has no longer single country’s affair, but is on the way to develop into a global pandemic requiring cooperation and control of all countries (32). The pandemic has so far spread across over 100 countries in the world. The global risk of pandemic spread means that the virus may spread to and originate from all directions. For most countries and regions, effective prevention and control are still “new topic” that has not been addressed in the previous pandemic outbreaks such as H1N1 or Ebola virus. In addition, there were large-scale gatherings in many highly infected countries such as South Korea at the beginning of the pandemic. Based on our modelling analysis of the impact of aggregation factors on the development of the pandemic, it is likely to see some European countries and the Middle East countries encounter similar risk of large-scale outbreak as South Korea, should no radical health policy be undertaken by the national governments in due course.
While the number of newly confirmed cases in China is declining, the current state in Italy and South Korea are particularly challenging as the number of infections have been keep increasing rapidly with over thousand new cases each day. Some severely impacted regions such as Daegu in South Korea and North Italy (Milan-Venice region) have already put in place draconian isolation measures within the urban areas. However, both our modelling results and the experience of Wuhan suggest that all non-necessary outdoor activities, in particular the social gathering and mobility should be strictly suspended immediately to minimise the interpersonal transmission likelihood. Also, external medical resources have to be mobilise nationwide to provide necessary medical support to the most infected areas. Further, temporary medical isolation centre may be built to increase the patient intake capacity in the event of serious outbreak. One of the main reasons of the high mortality rate of the infected patients in Wuhan during the early stage of the outbreak of COVID-19 was related to the saturation in local medical resources including overwhelmed treatment centres and lack of caring personnel. The situation only started to improve after the Chinese central government sent more than 50,000 medical staff to the city and more than 15 makeshift hospitals started operation to admit tens of thousands of patients with mild symptoms of novel coronavirus for the purpose of isolation. Thus, the treatment capacity is a determining factor in reducing the mortality of infected patients. This invaluable experience gained from China’s pandemic combat may be shared with the countries facing similar challenges.
Another critical issue in terms of lifecycle management of pandemic is related to the precautionary measures after the previously infected region or country has reached the stabilised state, e.g., all domestic infectious origins have been cleared off. In other words, how to minimize the risk of importing new cases for the disinfected origin of outbreak poses a serious challenge for local public health. This is particularly relevant to countries like China, with a large population and high degree of exposure to international travel, which is facing a high risk of imported cases. Any mishandling or improper prevention of the imported cases, a secondary transmission of novel coronavirus might lead to the outbreak anew3 (33).
Last, it should be pointed out that fluent information flow plays a central role in coordinating pandemic control activities of all stakeholders across the social network under risk through the whole process of pandemic control campaign. On the one hand, the timely release of information on the outbreak of infectious diseases is conducive to the prevention and control of infectious diseases in other provinces and regions. Clear and transparent information help correct understanding of new infectious diseases and pandemic situation of the public and stabilizing social mood on the other hand. Note that it is essential for health authorities to prevent the panic psychology from spreading across mass population when dealing with a public health crisis. To gain legitimacy and public trust in local authority and health care institutions, the golden triangle of punctuality, accuracy and transparency of pandemic related information should be consistently maintained at all times. The information and clinical experience sharing can also contribute to enhance the global capacity of containing the pandemic spread and minimising the life loss caused by the infections. As such, the interdisciplinary and intergovernmental coordination is a prerequisite of the success in combatting the COVID-19. The shared knowledge of global pandemic control parameters may provide useful lessons for countries on the set of increasing risks of large infections. It is imperative for global health decision makers to undertake timely countermeasures based on robust modelling of the likely occurrence of pandemic spread.
Acknowledgments
Funding: This work was partially supported by the National Natural Science Foundation of China [61973296] and Science and Technology Major Project of Tianjin Science and Technology Commission (Grant No. 20ZXGBSY00100).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1315
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-20-1315
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1315). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gilbert M, Pullano G, Pinotti F, et al. Preparedness and vulnerability of African countries against importations of COVID-19: a modelling study. Lancet 2020;395:871-7. [Crossref] [PubMed]
- Koopmans M. The Novel Coronavirus Outbreak: What We Know and What We Don’t. Cell 2020;180:1034-6. [Crossref] [PubMed]
- Coronavirus response: a focus on containment is still apt. Nature 2020;579:7. [Crossref] [PubMed]
- Watts CH, Vallance P, Whitty CJM. Coronavirus: global solutions to prevent a pandemic. Nature 2020;578:363. [Crossref] [PubMed]
- Yang Z, Zeng Z, Wang K, et al. Modified SEIR and AI prediction of the epidemics trend of COVID-19 in China under public health interventions. J Thorac Dis 2020;12:165-74. [Crossref] [PubMed]
- Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet 2020;395:689-97. [Crossref] [PubMed]
- World Health Organization (WHO). Coronavirus disease 2019 (COVID-19) Situation Report–48. Accessed on 09 March 2020. Available online: https://covid19.who.int/WHO-COVID-19-global-data.csv
- Hui DS, Azhar EI, Madani TA, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis 2020;91:264-6. [Crossref] [PubMed]
- Kupferschmidt K, Cohen J. Can China's COVID-19 strategy work elsewhere? Science 2020;367:1061-2. [Crossref] [PubMed]
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239-42. [Crossref] [PubMed]
- Ministry of Solidatrity, le service public de la diffusion du droit, France. Arrêté du 4 mars 2020: jusqu'au 31 mai 2020, tout rassemblement mettant en présence de manière simultanée plus de 5000 personnes en milieu closest interdit sur le territoire national. Accessed on 5 March 2020. Available online: https://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000041686833&dateTexte=&categorieLien=id
- Hellewell J, Abbott S, Gimma A, et al. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob Health 2020;8:e488-96. [Crossref] [PubMed]
- Codd EF. Cellular automata. New York: Academic Press, 1968.
- Zhao S, Lin Q, Ran J, et al. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: A data-driven analysis in the early phase of the outbreak. Int J Infect Dis 2020;92:214-7. [Crossref] [PubMed]
- White SH, Del Rey AM, Sánchez GR. Modeling epidemics using cellular automata. Appl Math Comput 2007;186:193-202. [Crossref] [PubMed]
- Holko A, Mȩdrek M, Pastuszak Z, et al. Epidemiological modeling with a population density map-based cellular automata simulation system. Expert Syst Appl 2016;48:1-8. [Crossref]
- dos Santos RMZ, Coutinho S. Dynamics of HIV infection: A cellular automata approach. Phys Rev Lett 2001;87:168102. [Crossref] [PubMed]
- Kim S, Byun JH, Jung IH. Global stability of an SEIR pandemic model where empirical distribution of incubation period is approximated by Coxian distribution. Adv Differ Equ 2019;2019:469. [Crossref]
- Page L, Brin S, Motwani R, et al. The PageRank citation ranking: Bringing order to the web. Stanford InfoLab 1999.
- Lai CC, Shih TP, Ko WC, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents 2020;55:105924. [Crossref] [PubMed]
- Heymann DL, Shindo N. WHO Scientific and Technical Advisory Group for Infectious Hazards. COVID-19: what is next for public health? Lancet 2020;395:542-5. [Crossref] [PubMed]
- Tang B, Wang X, Li Q, et al. Estimation of the transmission risk of the 2019-nCoV and its implication for public health interventions. J Clin Med 2020;9:462. [Crossref] [PubMed]
- Han Q, Lin Q, Jin S, et al. Coronavirus 2019-nCoV: A brief perspective from the front line. J Infect 2020;80:373-7. [Crossref] [PubMed]
- Benvenuto D, Giovanetti M, Ciccozzi A, et al. The 2019-new coronavirus epidemic: Evidence for virus evolution. J Med Virol 2020;92:455-9. [Crossref] [PubMed]
- Baharoon S, Memish ZA. MERS-CoV as an emerging respiratory illness: A review of prevention methods. Travel Med Infect Dis 2019;32:101520. [Crossref] [PubMed]
- Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun 2020;109:102433. [Crossref] [PubMed]
- Bai Y, Yao L, Wei T, et al. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA 2020;323:1406-7. [Crossref] [PubMed]
- Khoury MK, Heid CA, Cripps MW, et al. Antifungal Therapy in Fungal Necrotizing Soft Tissue Infections. J Surg Res 2020;256:187-92. [Crossref] [PubMed]
- Li JY, You Z, Wang Q, et al. The epidemic of 2019-novel-coronavirus (2019-nCoV) pneumonia and insights for emerging infectious diseases in the future. Microbes Infect 2020;22:80-5. [Crossref] [PubMed]
- World Health Organization (WHO). Infection prevention and control during health care when novel coronavirus (nCoV) infection is suspected: interim guidance, January 2020. Geneva: World Health Organization, 2020.
- TELLES CR. COVID-19, An overview about the pandemic virus behavior. MedRxiv 2020; published online Feb 24. Available online: https://doi.org/ [Crossref]
- Wang C, Horby PW, Hayden FG, et al. A novel coronavirus outbreak of global health concern. Lancet 2020;395:470-3. [Crossref] [PubMed]
- Sookaromdee P, Wiwanitkit V. Imported cases of 2019-novel coronavirus (2019-nCoV) infections in Thailand: Mathematical modelling of the outbreak. Asian Pac J Trop Med 2020;13:139-40. [Crossref]