Comparison of the outcomes of sublobar resection and stereotactic body radiotherapy for stage T1-2N0M0 non-small cell lung cancer with tumor size ≤ 5 cm: a propensity score matching analysis
Introduction
Lung cancer has more associated deaths than any other cancer globally (1), and its incidence rate is rising continuously. The widespread use of computed tomography (CT) for lung cancer screening has made detecting small peripheral pulmonary nodules possible (2,3). For operable patients with early-stage Non-small cell lung cancer (NSCLC), lobectomy with hilar and systematic lymph node evaluation is the standard treatment option (4,5). However, in some patients with older age or a high comorbidity burden, lobectomy is limited. For patients with localized, smaller, and peripheral tumors or higher comorbidity burden, or those with suspected lower tolerance for the loss of an entire pulmonary lobe, sublobar resection (SLR), which includes segmentectomy and wedge resection, has been considered (6-8).
SBRT is a novel radiation therapy modality, which delivers high-dose radiation to restricted volumes over a few fractions. With multiple, precisely aimed radiotherapy beams, the tumor can be ablated effectively with a lower quantity of irradiation to the surrounding normal tissue (9-13). Both the National Comprehensive Cancer Network Clinical Practice Guidelines and European Society for Medical Oncology Consensus recommend the use of SBRT as a non-surgical treatment option for stage I-II NSCLC (14). In our previously published study, the outcomes of surgery and SBRT on early-stage NSCLC were compared and no significant differences were found (15,16).
The comparative effectiveness of limited resection versus SBRT in patients who may tolerate surgical intervention, but not lobectomy, is still controversial (9-12). The development of nonsurgical therapies, such as SBRT and radiofrequency ablation have raised the issues of patient selection, treatment-related morbidity, and the relative oncologic efficacy. One report compared the short-term results among three prospective clinical trials using SLR [American College of Surgeons Oncology Group (ACOSOG) Z4032], stereotactic body radiotherapy (SBRT) [Radiation Therapy Oncology Group (RTOG) 0236] and radiofrequency ablation (ACOSOG Z4033) in the treatment of early stage NSCLC. The overall 90-day mortality for SLR, SBRT and radiofrequency ablation was 2.4%, 0% and 2.0% respectively (P=0.50), which creating an opportunity to examine alternative less-invasive therapies (13).
To date, few studies have specifically evaluated SLR and SBRT to guide us in selection of therapy for this challenging patient population. Therefore, we examined our institution’s experience with the use of SLR and SBRT to treat patients with T1-2N0M0 NSCLC by carrying out propensity score matching (PSM) analysis.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-2367).
Methods
Study population
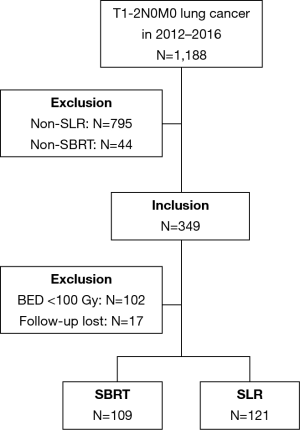
All procedures in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee of Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital) [NO.: IRB-2020-142 (KE)] and informed consent was taken from all the patients. All patients with pathologically or clinically confirmed T1-2N0M0 lung cancer who underwent SLR or SBRT at our institution between January, 2012 and December, 2016 were included. Clinically confirmed lung cancer was diagnosed by CT scan and was defined as a primary suspicious mass, part-solid, or ground-glass opacity nodule with spiculated or smooth margins that persisted for ≥3 months with an increase observed in its longest axis. In patients for whom the radiological results were inconclusive, endobronchial ultrasonography or mediastinoscopy was performed at the physician’s discretion. Bone scan and brain magnetic resonance imaging were sometimes needed for tumor staging. Positron emission tomography-CT (PET-CT) was recommended for each of the patients and utilized for the diagnosis if biopsy was not considered medically safe or the patient refused to undergo the procedure. The patients were staged according to the 7th edition of the Tumor, Node and Metastasis classification. Indications, technical aspects, complications, and impact on clinical outcomes were discussed by a dedicated multidisciplinary team. All multidisciplinary consultations were recorded in detail. Exclusion criteria included patients with pretreatment bulky lymph node (1 cm or more on a short axis) on chest CT, PET-CT positive lymph nodes, tumor diameter larger than 5 cm, multiple lung cancers, loss of follow-up, and those who have received non-SLR or SBRT with biological effective dose (BED) <100 Gy treatment. Details of inclusion or exclusion resulting in the final study group are described in the Figure 1.
Treatment procedures
When lobectomy was considered by the thoracic surgeon to be impractical for operable patients, SLR was performed via video-assisted thoracoscopic surgery under general anesthesia with single-lung ventilation via a double-lumen tracheobronchial tube. Wedge resection or segmentectomy was performed at the discretion of the surgeon and chosen prior to the procedure. The management included lobectomy associated with complete lymph node dissection through a conventional thoracotomy or a thoracoscopic approach, depending on the tumor depth and location and other characteristics of primary tumor. Each operation was performed with curative intent, and the tumor resection was confirmed by negative resection margins.
Patients who were medically inoperable because of comorbidities or because they rejected surgery were selected for SBRT. The entire course of SBRT was reported in our previous study (15,16). The definitions of the gross tumor volume (GTV), internal target volume (ITV), and planning target volume (PTV) are consistent with those in the American Society for Therapeutic Radiology and Oncology (ASTRO) guidelines (17). The BED was calculated using BEDα/β = nd (1+ d/α/β), in which n=number of fractions, d = dose per fraction, and α/β =10 Gy for the tumor (15,16).
Data collection
Patient characteristics and treatment complications
Demographic variables, which were obtained from records on the electronic database of the Cancer Hospital of the University of Chinese Academy of Sciences, included age, sex, pre-treatment pulmonary function test parameters, Karnofsky Performance Status score, and Charlson comorbidity index (CCI). Tumor characteristics included diameter, histology, grade, and location. Data of complications in the surgery group and toxicities in the SBRT group were collected and graded using the Common Terminology Criteria for Adverse Events Version 4.0. Within each category of adverse events, a description of severity or grade is included. Grade 1 is mild, grade 2, moderate, grade 3, severe, grade 4, life threatening or disabling, and grade 5, death related to the adverse event.
Patient follow-up
Post-treatment follow-up generally comprised a contrast-enhanced CT scan of the thorax and abdomen. The first patient follow-up took place within two months of the treatment being completed, then every three months for the first two years, and every six months thereafter. Primary tumor recurrence was diagnosed by histological confirmation or enlargement of the local tumor on CT that continued for ≥6 months. When recurrence was suspected, PET-CT was recommended. When PET-CT showed an intense uptake with a maximum standardized uptake value of ≥5 at ≥6 months after SBRT, recurrence was confirmed (18,19). Pulmonary fibrosis and tumor recurrence are difficult to distinguish; therefore, the post-SBRT imaging findings were reviewed by a senior radiologist, who to scored patterns of failure and eliminated historic discrepancies in the definitions of failure between surgery and SBRT. Locoregional failure was defined as disease recurrence within 2 cm of the original GTV (for SBRT patients) (20), resection margins (for surgery patients) (21), or the hilum, ipsilateral, or mediastinum regional lymph nodes. Distant failure indicated recurrence beyond locoregional failure.
Survival outcomes
Survival was compared between the SLR and SBRT groups. Disease-free survival (DFS) was defined as the time from the date of surgery or SBRT to the date of any failure or death, or the date of the last follow-up. Overall survival (OS) was defined as the time from the date of treatment was initiated to the date of death or last follow-up. Cancer-specific survival (CSS) was defined as the time from the date of treatment was initiated to the date of lung cancer or treatment-related mortality. Treatment-related toxicity was also analyzed.
PSM
The propensity score was calculated using multivariable logistic regression to model a dichotomous outcome of SLR or SBRT for the entire cohort of 230 patients. In an initial analysis, patients in the SLR and SBRT groups were compared based on age, gender, Karnofsky Performance Status score, CCI, tumor size, forced expiratory volume in 1 second (FEV1), forced vital capacity ratio (FEV1/FVC%), and predicted carbon monoxide diffusing capacity of the lung (DLCO%). The subjects were 1:1 matched by the estimated propensity score with a caliper of 0.09 of the logit of the propensity score (15,16).
Statistical analysis
The two-tailed t-test was used for continuous variables. Mann-Whitney U test was used to compare non-normally distributed data. Categorical variables were compared using the χ2 test. The Kaplan-Meier method was used to calculate the survival probability. PSM analysis was performed with the R MatchIt package (https://www.r-project.org/) for Windows. A two-tailed P value of <0.05 was taken to indicate statistical significance.
Results
Patient characteristics
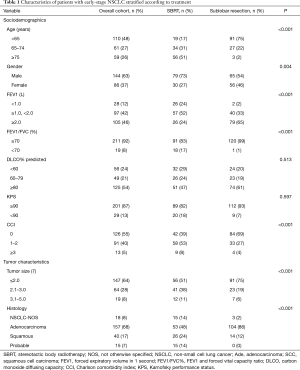
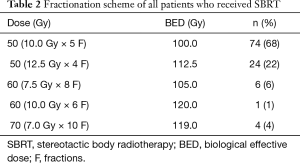
A total of 230 stage I NSCLC patients who had received either SLR (n=121) or SBRT (n=109) were selected for matching. Figure 1 shows the selection process of the study cohorts. Table 1 summarizes the baseline characteristics prior to PSM. In the SLR group, 64 patients (53%) had undergone wedge resection, while the remaining 57 patients (47%) had undergone segmentectomy. A total of 109 patients (90%) in the SBRT group had received video-assisted thoracoscopic surgery (VATS), and the remaining 10% had received open thoracotomy. The margins of the resection had been tumor-free in all cases. NSCLC had been histologically confirmed in 93% of the patients. Biopsy had not been performed for 15 patients in the SBRT group. PET-CT staging had been conducted for 72/109 patients (66%). The fractionation scheme mainly included 50 Gy in 4 fractions (n=24, 22%) or 50 Gy in 5 fractions (n=74, 68%). All of the patients included in our analysis had received a minimum BED10 of 100 Gy (range, 100–120 Gy). The SBRT dose used and the fractionation are detailed in Table 2.
Full table
Full table
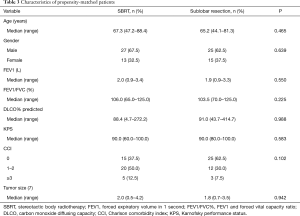
Each cohort comprised 40 patients, and balance was achieved based on the variables available (Table 3). Before PSM, patients in the SBRT group exhibited a significantly larger tumor size, poorer FEV1, higher CCI, and older age than those in the SLR group. The eligible patients had similar tumor sizes and respiratory function. Post-matching standardized differences for all measured covariates were <10%, which suggested substantial balance across the groups.
Full table
Survival
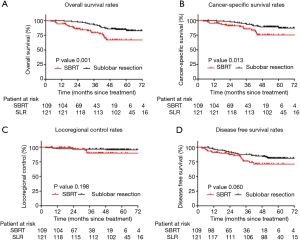
The average length of follow-up was 49.4 months (range, 3.9–76.4 months). Before PSM, the OS and CSS rates were superior in the SLR patients. The 5-year OS rate was 82.8% and 67.0% in the SLR and SBRT groups, respectively (P=0.001) (Figure 2A). The 5-year CSS rate was 89.0% and 75.3% in the SLR and SBRT groups, respectively (P=0.013) (Figure 2B). The unadjusted 5-year LRC and DFS rates were similar between the groups (95.7% vs. 89.9% and 81.0% vs. 71.2%, respectively) (Figure 2C,D).
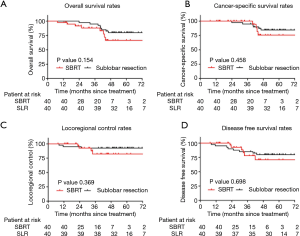
After PSM, eight patients died in each group during the follow-up period. Ten deaths (62.5%) were caused by cancer recurrence, and 6 cases (37.5%) were attributed to other causes. There were no differences noted between SLR and SBRT in terms of the 5-year OS rate (79.9% vs. 66.5%, respectively; P=0.154; Figure 3A) and the 5-year CSS rate (84.5% vs. 75.6%, respectively; P=0.458; Figure 3B). Notably, locoregional failure occurred in 3 and 4 cases in the SLR and SBRT groups, respectively. There was no significant difference in the LRC rate between the two groups (P=0.369). The 5-year LRC rates in the SLR and SBRT groups were 92.5% and 82.2%, respectively (Figure 3C). Distal failure occurred in 13 patients (i.e., 8 and 5 in the SLR and SBRT groups, respectively). Moreover, the 5-year DFS rate was 79.8% vs. 70.8%, respectively (P=0.698) (Figure 3D).
Treatment toxicity
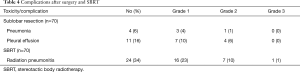
Table 4 outlines the complications which occurred after SLR and SBRT. After statistical adjustment, 11 patients (18%) experienced SBRT-associated adverse events within 6 weeks. The most commonly occurring adverse event was more severe or more frequent dyspnea, which was reported by 55% of patients. One patient suffered grade 3 radioactive pneumonitis, according to the RTOG (22). No grade 4 or 5 toxicities were reported. Systemic reactions during treatment, including fatigue, anorexia, and dyspnea, resolved after symptomatic treatment. In the SLR group, 15 (22%) patients suffered complications. Grade 2 complications were reported in 5 cases (33%). None of the patients suffered grade >3 toxicities or treatment-related death.
Full table
Discussion
The widespread introduction of low-dose spiral CT for population screening may lead to a rise the number of early-stage NSCLC patients (2,3). Recently, interest in the use of less extensive and hence, more lung-sparing surgery, such as SLR, has increased for the treatment of older patients and those with more advanced systemic medical conditions (23). Non-surgical approaches, including SBRT, may indeed represent an attractive alternative. However, research evaluating SLR and SBRT is currently limited, and there is minimal information to guide clinicians. The randomized trial investigating SLR and SBRT (ACOSOG Z4099/RTOG 1021) have been closed by poor enrollment. Only the STABLE-MATES study (NCT02468024) directly compared SLR with SBRT, which is often the only resection approach that can be tolerated by elderly patients with comorbidities. The primary endpoint is 3-year OS, and the expected study completion date is 12-2024.
Given the considerable challenges in executing randomized trials, controlled population-based analyses may supply important evidence in this setting. Our single-institution, retrospective study compared SBRT with SLR, a popular option among surgeons for patients whose heart and lung function means they are considered to be unable to tolerate pneumonectomy. Unmatched analyses in the present study had shown higher 5-year OS and CSS rates after SLR. This finding may be attributed to the patient characteristics being unequally distributed. Patients with a higher comorbidity burden and worse respiratory function, for instance, tended to be treated with SBRT. We subsequently performed a matched-pair analysis to match patients and tumor-related characteristics. In this way, PSM can reduce potential confounding bias at baseline, and so patients with similar distributions could be identified, approximating a randomized controlled trial. The results indicated there to be no significant differences in the OS or CSS rates between the SLR and SBRT groups. Furthermore, as expected, the differences between the LRC and DFS rates were also not significant. It must be pointed out that due to the limited sample size of each group after statistical adjustment, the above results must be treated cautiously. However, these observations are generally consistent with those previously reported, which indicates that our results hold a certain degree of significance (9-11,24,25). The outcomes of this study may assist in forming the basis of future trials for the comparison of these two interventions.
Despite the excellent LRC rate achieved by modern SBRT fractionation regimens, we found that distant failure was the most common type of recurrence after radiotherapy, with a similar 3-year rate (20.0%) to those reported in previous studies. In RTOG 0236, 15 of 59 patients developed distant recurrence (20). Similarly, the Japan Clinical Oncology Group (0403) demonstrated that distant recurrence is still the dominant type of failure, accounting for the majority (23/31) of recurrences involving a component at distant metastatic sites (26). Given PET-CT and endobronchial ultrasound transbronchial needle aspiration are less sensitive to nodal metastasis compared to nodal dissection, these findings are not impressive (27,28). To reduce the risk of metastasis, some patients need effective adjuvant therapy.
Because long-term survival after treatment of early-stage NSCLC is possible, toxicity has become especially important. In this study, there was only a limited amount of toxicity observed in the two groups. This finding corresponds with those of previous study (29,30). For high-risk operable patients, SBRT appears to be an attractive option as a curative modality for early-stage NSCLC. However, several factors should be considered in this comparison of outcomes. Most surgical complications are acute or subacute, occurring within the first 30 to 90 days. Some complications of SBRT, such as rib fracture or a decline in pulmonary function, may occur several months or years after radiotherapy and were not accounted for with this short-term analysis. Most importantly, these short-term data should ultimately be compared within the context of the relative efficacy of the treatment modality on OS and DFS after longer follow-up.
There are several limitations to our study. Firstly, this study was based on a retrospective review of patient information; thus, inherent selection bias exists. For lower-risk patients, surgery may have been preferable. Secondly, the SBRT-treated patients tended to be clinically staged, whereas the patients who underwent SLR were ultimately pathologically staged. As most SBRT cases did not undergo nodal staging or dissection in our analysis, these patients might be underestimated, resulting in a pathological staging bias favoring surgery. Furthermore, our sample size after PSM was relatively small, and the statistical power was not robust enough. Thus, there was limited precision in the estimation of significant differences in outcomes across the treatment groups. Therefore, the results of the present study should be interpreted carefully.
The application of SBRT as a definitive treatment for NSCLC staged as curable with surgery has been controversial (31). In the absence of robust prospective data to guide management, rational clinical decision-making is required when recommending either SLR or SBRT for patients with early-stage NSCLC who are considered as marginal or poor operative candidates. Given the ambiguity of the proper use of SBRT in patients at high risk for lobectomy, randomized controlled trials are urgently needed to obtain and verify evidence based on the introduction of SBRT into clinical practice.
Conclusions
The management of high-risk operable patients who cannot tolerate lobectomy due to medical comorbidities poses a unique challenge. Our results suggest that SBRT achieved comparable overall clinical outcomes as those reported with SLR in the PSM analysis cohort. These data may provide healthcare providers with a reference for decision-making for early-stage NSCLC patients who are borderline operable, or at a high risk of perioperative mortality.
Acknowledgments
We thank the patients for their time and participation in this study.
Funding: This work was supported by the grants from the Zhejiang province public welfare funds (No. GF20H160009) and the Medical Science and Technology Project of Zhejiang Province (No. 2020382901).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-2367
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-2367
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-2367). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee of Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital) (NO.: IRB-2020-142 (KE)) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015;385:977-1010. [Crossref] [PubMed]
- Qian F, Yang W, Chen Q, et al. Screening for early stage lung cancer and its correlation with lung nodule detection. J Thorac Dis 2018;10:S846-S859. [Crossref] [PubMed]
- Weber M, McWilliams A, Canfell K. Prospects for cost-effective lung cancer screening using risk calculators. Transl Cancer Res 2019;8:S141-4. [Crossref]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-e313S.
- Ahn S, Jeong JY, Kim HW, et al. Robotic lobectomy for lung cancer: initial experience of a single institution in Korea. Ann Cardiothorac Surg 2019;8:226-232. [Crossref] [PubMed]
- Fernandez FG, Kosinski AS, Burfeind W, et al. The Society of Thoracic Surgeons Lung Cancer Resection Risk Model: Higher Quality Data and Superior Outcomes. Ann Thorac Surg 2016;102:370-7. [Crossref] [PubMed]
- McMurry TL, Shah PM, Samson P, et al. Treatment of stage I non-small cell lung cancer: What's trending? J Thorac Cardiovasc Surg 2017;154:1080-7. [Crossref] [PubMed]
- Ferrari-Light D, Cerfolio RJ. Non-small cell lung cancer 2 cm or less: robotic segmentectomy sets the gold standard against non-surgical therapy. Ann Transl Med 2019;7:S96. [Crossref] [PubMed]
- Ackerson BG, Tong BC, Hong JC, et al. Stereotactic body radiation therapy versus sublobar resection for stage I NSCLC. Lung Cancer 2018;125:185-91. [Crossref] [PubMed]
- Paul S, Lee PC, Mao J, et al. Long term survival with stereotactic ablative radiotherapy (SABR) versus thoracoscopic sublobar lung resection in elderly people: national population based study with propensity matched comparative analysis. BMJ 2016;354:i3570. [Crossref] [PubMed]
- Matsuo Y, Chen F, Hamaji M, et al. Comparison of long-term survival outcomes between stereotactic body radiotherapy and sublobar resection for stage I non-small-cell lung cancer in patients at high risk for lobectomy: A propensity score matching analysis. Eur J Cancer 2014;50:2932-8. [Crossref] [PubMed]
- Tamura M, Matsumoto I, Tanaka Y, et al. Comparison between Stereotactic Radiotherapy and Sublobar Resection for Non-Small Cell Lung Cancer. Ann Thorac Surg 2019;107:1544-50. [Crossref] [PubMed]
- Crabtree T, Puri V, Timmerman R, et al. Treatment of stage I lung cancer in high-risk and inoperable patients: comparison of prospective clinical trials using stereotactic body radiotherapy (RTOG 0236), sublobar resection (ACOSOG Z4032), and radiofrequency ablation (ACOSOG Z4033). J Thorac Cardiovasc Surg 2013;145:692-9. [Crossref] [PubMed]
- Vansteenkiste J, Crinò L, Dooms C, et al. 2nd ESMO Consensus Conference on Lung Cancer: early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann Oncol 2014;25:1462-74. [Crossref] [PubMed]
- Dong B, Wang J, Xu Y, et al. Comparison of the Efficacy of Stereotactic Body Radiotherapy versus Surgical Treatment for Early-Stage Non-Small Cell Lung Cancer after Propensity Score Matching. Transl Oncol 2019;12:1032-7. [Crossref] [PubMed]
- Dong B, Wang J, Zhu X, et al. Comparison of the outcomes of stereotactic body radiotherapy versus surgical treatment for elderly (≥70) patients with early-stage non-small cell lung cancer after propensity score matching. Radiat Oncol 2019;14:195. [Crossref] [PubMed]
- Videtic GMM, Donington J, Giuliani M, et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive Summary of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol 2017;7:295-301. [Crossref] [PubMed]
- Matsuo Y, Nakamoto Y, Nagata Y, et al. Characterization of FDG-PET images after stereotactic body radiation therapy for lung cancer. Radiother Oncol 2010;97:200-4. [Crossref] [PubMed]
- Huang K, Dahele M, Senan S, et al. Radiographic changes after lung stereotactic ablative radiotherapy (SABR)--can we distinguish recurrence from fibrosis? A systematic review of the literature. Radiother Oncol 2012;102:335-42. [Crossref] [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Grills IS, Mangona VS, Welsh R, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol 2010;28:928-35. [Crossref] [PubMed]
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995;31:1341-6. [Crossref] [PubMed]
- Donington JS. Point: are limited resections appropriate in non-small cell lung cancer? Yes. Chest 2012;141:588-90. [Crossref] [PubMed]
- Shirvani SM, Jiang J, Chang JY, et al. Comparative effectiveness of 5 treatment strategies for early-stage non-small cell lung cancer in the elderly. Int J Radiat Oncol Biol Phys 2012;84:1060-70. [Crossref] [PubMed]
- Shirvani SM, Jiang J, Chang JY, et al. Lobectomy, sublobar resection, and stereotactic ablative radiotherapy for early-stage non-small cell lung cancers in the elderly. JAMA Surg 2014;149:1244-53. [Crossref] [PubMed]
- Nagata Y, Hiraoka M, Shibata T, et al. Prospective Trial of Stereotactic Body Radiation Therapy for Both Operable and Inoperable T1N0M0 Non-Small Cell Lung Cancer: Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol Biol Phys 2015;93:989-96. [Crossref] [PubMed]
- Ong P, Grosu H, Eapen GA, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for systematic nodal staging of lung cancer in patients with N0 disease by computed tomography and integrated positron emission tomography-computed tomography. Ann Am Thorac Soc 2015;12:415-9. [Crossref] [PubMed]
- Fernandez FG, Kozower BD, Crabtree TD, et al. Utility of mediastinoscopy in clinical stage I lung cancers at risk for occult mediastinal nodal metastases. J Thorac Cardiovasc Surg 2015;149:35-41, 42.e1.
- Varlotto J, Fakiris A, Flickinger J, et al. Matched-pair and propensity score comparisons of outcomes of patients with clinical stage I non-small cell lung cancer treated with resection or stereotactic radiosurgery. Cancer 2013;119:2683-91. [Crossref] [PubMed]
- Iguchi T, Hiraki T, Matsui Y, et al. Survival Outcomes of Treatment with Radiofrequency Ablation, Stereotactic Body Radiotherapy, or Sublobar Resection for Patients with Clinical Stage I Non-Small-Cell Lung Cancer: A Single-Center Evaluation. J Vasc Interv Radiol 2020;31:1044-51. [Crossref] [PubMed]
- Rice D, Sepesi B, Heymach J, et al. SABR vs surgery for NSCLC in the media. Lancet Oncol 2015;16:e422. [Crossref] [PubMed]
(English Language Editor: J. Reynolds)