The clinical suspicion of a leaking intrathoracic esophagogastric anastomosis: the role of CT imaging
Introduction
Esophageal cancer is the 8th most common malignancy and 6th leading cause of cancer-related mortality worldwide. Annually, more than 450,000 new esophageal cancer cases and 400,000 cancer deaths are estimated (1). Trimodality therapy, consisting of neoadjuvant chemoradiotherapy and esophagectomy, represents the gold standard for potentially curable esophageal cancer (2). Esophagectomy with the creation of an intrathoracic anastomosis is increasingly applied (3) and might reduce anastomotic leakage incidence compared to a cervical anastomosis (4). In the Netherlands, the incidence of intrathoracic anastomotic leakage is approximately 18% and remains the primary source of morbidity and mortality (5,6). Anastomotic leakage is frequently accompanied by other complications and adversely impact survival rates (7), recurrence of disease (8) and is associated with an extended postoperative period, leading to a substantial increase in hospital costs (9,10). These adverse effects may be averted if anastomotic leakage is diagnosed early and intervened properly.
Clinical presentations of anastomotic leakage are diverse and varies from asymptomatic to severely septic patients. Clinical distinction between anastomotic leakage and other complications such as pneumonia and systemic inflammatory response syndrome (SIRS) is challenging as clinical symptoms are near to identical (11,12). Therefore, leakage is mainly detected using a combination of clinical, endoscopic and radiological evaluation. Assessment of thoracic computed tomography (CT) images, especially during the early postoperative period, may be challenging. The presence of mediastinal air or fluid collections may indicate leakage out of the gastric conduit, but may also be a normal postoperative finding (13). These challenges are also encountered following other gastrointestinal surgical procedures and previous studies have found several radiological findings useful for the prediction of anastomotic leakage following colorectal, pancreatic and gastric surgery (14,15).
Objective of this study was to determine the diagnostic value of CT in case of suspected anastomotic leakage. A secondary objective was to identify reliable CT findings predicting anastomotic leakage. Based on these findings a practical scoring system was proposed in order to aid radiologists and surgeons in the systematic assessment of CT scans. This scoring system may facilitate objective interpretation of CT findings to confirm or rule out anastomotic leakage.
Methods
Study population
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Medical Ethical Review Committee of the VU University Medical Center (2016.480) and by the local institutional ethical review board of the Catharina Hospital.
This study was designed as a multicenter retrospective cohort study. All consecutive patients underwent a CT scan for a clinical suspicion of anastomotic leakage following Ivor Lewis (16) esophagectomy between October 2010 and July 2016 in the VU University Medical Center in Amsterdam or in the Catharina Hospital in Eindhoven. The surgical procedure consisted of a two-stage laparoscopic and thoracoscopic esophagectomy with en-bloc lymphadenectomy, the formation of a gastric conduit and the creation of a thoracic esophagogastric anastomosis. The anastomosis was created in an end-to-side fashion using 25 mm circular stapling or end-to-side/side-to-side using linear stapling and sutured closure of the stapling defect. CT imaging was performed if patients were suspect of anastomotic leakage within the first 30 days after surgery, based on clinical examination of the patient and laboratory findings. To prevent bias in assessment of leakage of contrast, scans were excluded when oral contrast was not (correctly) admitted. This study was reported according to The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting checklist (17) (available at http://dx.doi.org/10.21037/jtd-20-954).
Image acquisition
Thoraco-abdominal CT images were acquired using commercially available 16-, 64- or 128-section CT scanners (Philips Medical Systems, Best, The Netherlands and Siemens, Erlangen, Germany). Images were typically acquired with 16×0.9, 64×0.625 or 128×0.625 mm section collimation, a tube rotation time of 330–750 ms, a tube potential of 100 or 120 kV, an effective tube current ranging from of 20–1,000 mAs and a pitch of 0.7, 0.9 or 1.1. An iodinated 70–100 mL contrast material bolus (300 mg/mL) was administered intravenously at 2 to 4 mL/sec in all patients. Scan delay ranged from 25/30 and 60/70 seconds after injection of contrast. Patients received 200 mL iodinated oral contrast media (Telebrix Gastro, 300 mgI/mL) in the first hour prior to CT imaging, followed by 200 mL just five minutes before imaging.
Parameter selection and definitions
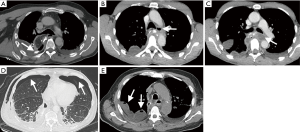
CT findings of interest were selected prior to the study. A gastrointestinal surgeon and radiologist independently evaluated 20 CT scans of patients following Ivor Lewis esophagectomy, consisting of 10 healthy postoperative patients and 10 patients with known anastomotic leakage. The following CT findings were selected in consensus: extraluminal oral contrast, visible wall discontinuity, air collection at the anastomotic site (located 2 cm proximal or distal to the anastomosis), mediastinal air collection, air collection below the diaphragm, subcutaneous emphysema, pneumothorax, fluid collection at the anastomotic site (located 2 cm proximal or distal to the anastomosis), mediastinal fluid collection, loculated pleural effusion (pocket of or trapped pleural fluid) and infiltration of fat at the anastomotic site (non-contiguous inhomogeneity with low tissue density, located 2 cm proximal or distal to the anastomosis).
Review process
All CT images were randomized and independently assessed by two experienced gastrointestinal radiologists (A and B), who were blinded for clinical information. The presence or absence of the predetermined CT findings was systematically documented. The radiologists finalized the assessment by stating whether anastomotic leakage was suspected (classified as “no anastomotic leakage” or “suspected anastomotic leakage”), further referred to as “blinded interpretation of the radiologists”. Following blinded assessment by the two radiologists, the original CT reports were re-evaluated and conclusions were classified as “no anastomotic leakage”, “suspicion of anastomotic leakage” or “definite anastomotic leak”.
Anastomotic leakage
Anastomotic leakage was clinically suspected based on clinical deterioration, elevated serum c-reactive protein and/or abnormal drain production. Clinical deterioration was defined by a combination of tachycardia (>100 beats per minute), tachypnea (ventilation rate of >20 per minute), hypotension (systolic pressure <100 mmHg) or fever (>38 °C). Serum c-reactive protein levels of >140 mg/dL were considered elevated. When anastomotic leakage was clinically suspected, a CT scan was requested to objectify. In this study, leakage was later confirmed by visualization of the defect during endoscopy or thoracotomy. Severity was graded based on required treatment according to definitions stated by the Esophagectomy Complications Consensus Group (ECCG) (18). Additional patient, surgery and pathology data were registered.
Statistical analysis and scoring system
For continuous data, independent sample t tests or Mann-Whitney U tests were used. Dichotomous and categorical variables were analyzed using Chi-Square tests or Fisher’s exact tests (in case of small cell counts). The level of inter-observer agreement between radiologists was derived from Kappa values and defined as follows: 0–0.20 poor agreement, 0.21–0.40 fair agreement, 0.41–0.60 moderate agreement, 0.61–0.80 substantial agreement and 0.81–1 excellent agreement. The original report and blinded interpretation of the radiologists were used as reference standard and their diagnostic performances were computed by means of cross tabulation and receiver operating characteristics (ROC). Univariable logistic regression was used to determine the association between individual CT findings and anastomotic leakage, providing odds ratios (OR) with 95% confidence intervals (CI) and p-values. Subgroup analyses were performed to assess whether timing of CT imaging [postoperative day (POD) 1–4 vs. POD 5–30] influenced the associations of the different CT findings with anastomotic leakage. Multivariable backward stepwise logistic regression of CT findings was performed to develop a CT-based practical scoring system. CT findings with a p-value of <0.157, based on the Akaike information criterion (19), retained in the scoring system. Based on the beta-regression coefficients of the retained CT findings, previous research (13,14) and expert opinion the importance of each CT finding was determined and converted into an amount of points. A higher amount of points indicated a higher predicted probability of anastomotic leakage. The diagnostic performance was determined by ROC curve analysis, and calculation of the area under the receiver operating characteristics (AUROC). Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were computed for each amount of points. The diagnostic accuracy of the scoring system was compared to the blinded interpretation of the radiologists and original reports (reference standard). Radiologist A had a superior diagnostic accuracy over radiologist B and therefore the CT assessment of radiologist A was used for logistic regression analysis. IBM SPSS statistics (version 23) was used for standard statistical analysis and a P value of <0.05 was considered statistically significant.
Results
Patient characteristics
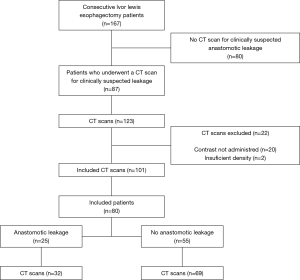
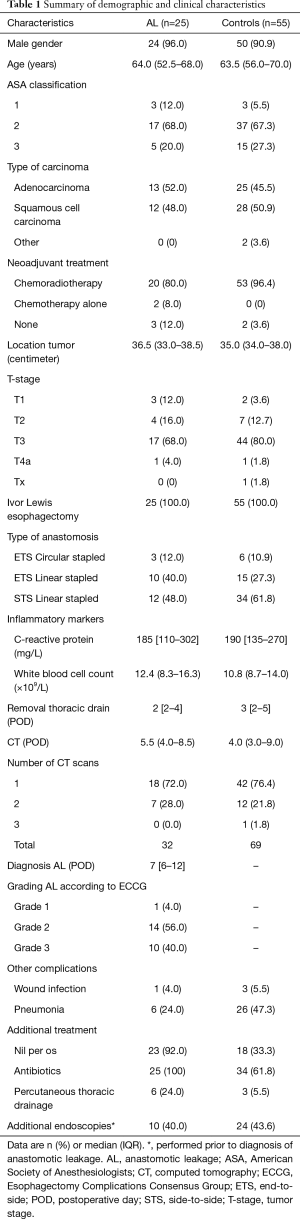
In our study population, 167 patients underwent esophagectomy with an intrathoracic anastomosis (Figure 1). Of these patients, 87 had clinical signs of anastomotic leakage and one or multiple CT scans were performed. Seven patients were excluded (22 CT scans) due to insufficient density of oral contrast or oral contrast was omitted, resulting in 80 patients and a total of 101 CT scans (Figure 1). Median POD of the scans was day 4 [inter quartile range (IQR): 3–9]. Further subject characteristics are summarized in Table 1, no statistically significant differences were found between groups.
Full table
Anastomotic leakage
Thirty-two CT scans were performed in 25 patients with a confirmed anastomotic leak. Median POD to clinical diagnosis of anastomotic leakage was day 9 (IQR, 6–12). Initially, leakage was diagnosed by CT imaging (15 patients) or, when required, by additional endoscopy (9 patients), diagnostic thoracotomy (1 patient). Leakage was subsequently confirmed by a therapeutic endoscopy (15 patients) or thoracotomy (10 patients). Endoscopic stent placement was not feasible in one patient and was treated with antibiotic therapy, nasogastric tube drainage and total parenteral nutrition. This resulted in one patient with a grade 1 anastomotic leak, 14 patients with grade 2 anastomotic leaks and finally 10 patients with a grade 3 leak [grading according to ECCG (18)].
Multiple CT scans
In 20 patients multiple CT scans [two CT scans (n=19) or three CT scans (n=1)], were required to diagnose or rule out anastomotic leakage. The first scans (median POD 4) reported no anastomotic leak (n=18) or suspected leak (n=2). Seven patients underwent an additional endoscopy in which no leaks were seen. All patients were closely monitored and when the suspicion of anastomotic leakage remained, a second CT scan was requested to identify new findings (median POD 12). The second CT scan concluded a clear anastomotic leak (n=4), suspected leak (n=3) or no leak (n=13). Additional diagnostic endoscopies were performed in 8 patients, which revealed an anastomotic leak in three patients, and no apparent leak in five patients. In one patient a third CT scan (POD 22) reported a suspected leak, however additional endoscopy revealed no leak in this patient.
Inter-observer agreement
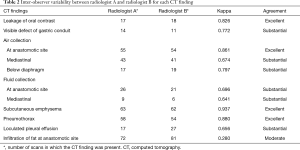
The results of the Cohen’s kappa measures for both radiologists are displayed in Table 2. Overall, the inter-observer agreement between the two radiologists was substantial to excellent for most CT findings. The inter-observer agreement of the presence of surrounding tissue infiltration was moderate with a Kappa value of 0.280. The rest of the findings had a Kappa value of 0.641 or more.
Full table
Predictive CT findings
The individual CT findings and their association with postoperative anastomotic leakage are displayed in Table 3. Univariable logistic regression analysis showed that the presence of extraluminal oral contrast (OR: 19.41, 95% CI: 5.04–74.83, P<0.001), visible wall discontinuity (OR: 5.01, 95% CI: 1.52–16.51, P=0.008), air collection at the anastomotic site (OR: 10.89, 95% CI: 3.44–34.52, P<0.001), mediastinal air collection (OR: 5.03, 95% CI: 2.03–12.45, P<0.001), fluid collection at the anastomotic site (OR: 3.69, 95% CI: 1.45–9.42, P=0.006), mediastinal fluid collection (OR: 5.08, 95% CI: 1.18–21.82, P=0.029), pneumothorax (OR: 3.09, 95% CI: 1.22–7.82, P=0.017), loculated pleural effusion (OR: 5.50, 95% CI: 1.81–16.70, P=0.003) and infiltration of fat at anastomotic site (OR: 5.85, 95% CI: 1.62–21.12, P=0.007) were significantly associated with the occurrence of anastomotic leakage.

Full table
Subgroup analysis was performed for early CT scans (POD 1-4) and late CT scans (POD 5–30). The early group contained 54 scans, anastomotic leakage was confirmed in 16 scans and the late group consisted of 47 scans in which a leak was confirmed in 16 CT scans. These differences were not significant (P=0.634). In patients without anastomotic leakage subcutaneous emphysema (81.6% vs. 41.9%, P=0.001) and air collections below the diaphragm (26.3% vs. 0.0%, P=0.002) were more frequently observed in early compared to late scans. No significant differences in CT-scan findings were observed between early and late CT-scans in patients that suffered anastomotic leakage.
Scoring system
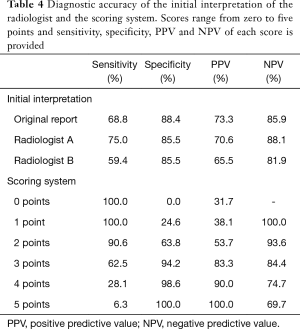
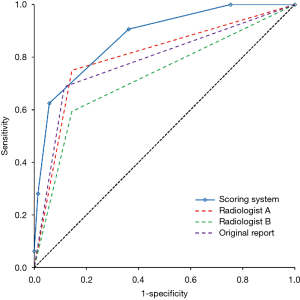
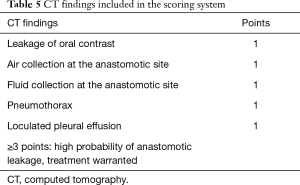
Multivariable backward stepwise logistic regression analysis was performed and all individual CT findings were included in the initial analysis. After multiple rounds the following findings retained in the model: presence of extraluminal oral contrast (β coefficient: 1.688, OR: 5.41, 95% CI: 1.13–25.79, P=0.034), air collection at the anastomotic site (β coefficient: 1.744, OR: 5.72, 95% CI: 1.46–22.41, P=0.012), fluid collection at the anastomotic site (β coefficient: 1.434, OR: 4.20, 95% CI: 1.08–16.38, P=0.039), pneumothorax (β coefficient: 1.841, OR: 6.30, 95% CI: 1.55–25.62, P=0.010), and loculated pleural effusion (β coefficient: 2.336, OR: 10.34, 95% CI: 1.53–70.07, P=0.017). It was decided that all characteristics were given one point each (Table 4, Figure 2), resulting in a cumulative score ranging from 0 to 5. The 5-point scoring system was applied to the entire dataset, resulting in an observed AUROC of 0.875 (95% CI: 0.802–0.947). The ROC was compared to the original report and blinded interpretation of the radiologists and represented in Figure 3. The sensitivity, specificity, PPV and NPV for each score is displayed in Table 5. A score of 3 or more points was statistically determined as the optimal cut-off and yielded a sensitivity of 62.5%, specificity of 94.2%, PPV of 83.3% and NPV of 84.4%.
Full table
Full table
Diagnostic value of CT imaging
The original CT reports concluded no anastomotic leakage in 71 scans, of which 10 had a confirmed anastomotic leak (NPV: 85.9%). An anastomotic leak was suspected in 30 scans (16 definite leaks and 14 suspected leaks), which was confirmed in 22 cases (PPV: 73.3%). This resulted in an AUROC of 0.786 (95% CI: 0.680–0.891) and a corresponding sensitivity of 68.8% and specificity of 88.4%. Radiologists A and B suspected anastomotic leakage in 34 and 29 scans, which appeared to be the case in 24 and 19 scans (PPV: 70.6% and 65.5% respectively). The absence of leakage was scored in 67 and 72 scans of which 8 and 13 had a confirmed leak (NPV: 88.1% and 81.9% respectively). This resulted in AUROC of 0.803 (95% CI: 0.702–0.903) for radiologist A (sensitivity: 75.0% and specificity: 85.5%) and 0.724 (95% CI: 0.610–0.724) for radiologist B (sensitivity: 59.4% and specificity: 85.5%). The diagnostic performance of the original report and blinded interpretation of the radiologists are summarized in Table 5, the corresponding ROC curve analyses are displayed in Figure 3.
Discussion
This study reported the diagnostic accuracy of CT imaging in patients who were clinically suspected of having a postoperative anastomotic leak. A sensitivity ranging from 59.4% to 75.0% and a specificity ranging from 85.5% to 88.4% was observed. Additionally, CT findings were systematically analyzed and predictors of anastomotic leakage were identified. This resulted in a practical 5-point scoring system involving the following CT findings: extraluminal oral contrast, air collection at the anastomotic site, fluid collection at the anastomotic site, pneumothorax and loculated pleural effusion. A score of 3 points was determined as optimal cut-off, in which patients with 3 or more points were considered at high risk of anastomotic leakage, warranting additional endoscopic evaluation to confirm the diagnosis, evaluate the size of the defect or initiate early treatment. Patients with 2 or even less points were considered at low risk for anastomotic leakage, for these patients a wait and see strategy is justified.
To date, this study is the largest of two studies investigating individual CT findings for the detection of intrathoracic anastomotic leakage. Upponi et al. (20) showed that air and fluid collections in the mediastinum had a predictive value in the assessment of anastomotic leakage. Strauss et al. (21) observed that the presence of contrast leakage and mediastinal air collections were associated with anastomotic leakage. The current study not only confirms the data in a larger cohort, but developed a clinically applicable scoring system for the detection of anastomotic leakage. Goense et al. (13) made a similar effort to develop a CT-based risk score for cervical leaks. Cervical leakage is associated with other CT findings compared to intrathoracic leaks and was therefore not our interest. In current study, the presence of extraluminal oral contrast, loculated pleural effusion and anastomotic or mediastinal air/fluid collections were registered.
Besides CT imaging with oral contrast, routine contrast swallow radiography has been suggested to assess anastomotic integrity. Multiple studies have been conducted demonstrating similar specificities for both examinations. Overall, CT imaging is associated with a higher sensitivity compared to radiography (20-22). Additionally, CT imaging has the added benefit of being easier to perform in critically ill patients, allows for recognition of associated findings (e.g., pleural effusion, pneumothorax, pulmonary abnormalities or undrained fluid collections) and can be performed by radiologic technologists. On the other hand, the obligatory delay in CT scanning following oral contrast administration could lead to increased false negative results for subclinical grade 1 leaks, compared to real-time radiography.
Endoscopic evaluation of the anastomosis provides excellent visibility of the surgical site and remains the golden standard in case of inconclusive imaging (23). On the other hand, aspiration, iatrogenic injuries and deterioration of the anastomotic dehiscence by inflating the esophagus remain a concern. When clinical findings are indicative for anastomotic leakage, CT imaging is justified as first diagnostic approach, followed by endoscopic evaluation in case of uncertainty.
In this study, the inter-observer agreement between the radiologists proved to be substantial to excellent for the findings included in the scoring system, yet there was a difference in their interpretation. The accuracy for predicting leakage of radiologist A was substantially better compared to radiologist B (AUROC: 0.803 and 0.724 respectively), indicating that, even when assessment of individual CT findings is accurate, interpretation remains challenging. As a result, 10 out of 25 patients with anastomotic leakage required an additional endoscopy or thoracotomy to diagnose the anastomotic leak. The proposed scoring system could serve as a guide for radiologists and surgeons in the systematic assessment of CT scans and prediction of anastomotic leakage. Further validation is needed to solidify its diagnostic accuracy amongst other radiologists and surgeons.
After multivariable analysis, loculated pleural effusion was the strongest predictor for anastomotic leakage, indicated by its superior beta regression coefficient. Extraluminal oral contrast showed to be the strongest individual predictor. Yet, both findings received one point in the scoring system. Assigning 2 or more points to the presence of extraluminal oral contrast or loculated pleural effusion did not improve the discriminative ability of the scoring system. This can be attributed to the observation that both findings were hardly reported without the presence of multiple other findings included in the scoring system.
This study is subject to potential limitations. First of all, some subclinical grade 1 leaks might have been missed as only not all patients underwent a postoperative endoscopy in that group. Furthermore, CT imaging might be less effective in detecting small grade 1 leaks due to the obligatory delay between scanning and oral contrast administration. Second, most patients with anastomotic leakage in our cohort underwent either a therapeutic endoscopy or thoracotomy (grade 2 and 3), which makes it difficult to extrapolate the diagnostic accuracy of the scoring system to early grade 1 anastomotic leaks. Finally, the scoring system needs to be externally validated by different radiologists on another cohort of patients.
Literature shows that laboratory findings of increased inflammatory markers can be predictive for anastomotic leakage (24,25). Imputing these with CT findings other factors, such as clinical symptoms and vital parameters, could render a diagnostic algorithm with a very high post-test probability. Further prospective research should focus on developing such an algorithm and determine its clinical value by comparing it to current practice.
In conclusion, this study provides an easy to use 5-point scoring system with good diagnostic accuracy and low inter-observer variability for the assessment of the anastomotic integrity after Ivor Lewis esophagectomy. A score of 3 or more points indicated a high probability of anastomotic leakage, justifying early initiation of treatment or additional endoscopic evaluation. Patients with lower scores are considered at low risk for anastomotic leakage, however vigilant assessment remains warranted.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-954
Data Sharing Statement: http://dx.doi.org/10.21037/jtd-20-954
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-954). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Medical Ethical Review Committee of the VU University Medical Center (2016.480) and by the local institutional ethical review board of the Catharina Hospital.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Thrift AP. The epidemic of oesophageal carcinoma: Where are we now? Cancer Epidemiol 2016;41:88-95. [Crossref] [PubMed]
- Biere SS, Maas KW, Cuesta MA, et al. Cervical or thoracic anastomosis after esophagectomy for cancer: a systematic review and meta-analysis. Dig Surg 2011;28:29-35. [Crossref] [PubMed]
- Goense L, Meziani J, Ruurda JP, et al. Impact of postoperative complications on outcomes after oesophagectomy for cancer. Br J Surg 2019;106:111-9. [Crossref] [PubMed]
- Gooszen JAH, Goense L, Gisbertz SS, et al. Intrathoracic versus cervical anastomosis and predictors of anastomotic leakage after oesophagectomy for cancer. The Br J Surg 2018;105:552-60. [Crossref] [PubMed]
- Rizk NP, Bach PB, Schrag D, et al. The impact of complications on outcomes after resection for esophageal and gastroesophageal junction carcinoma. J Am Coll Surg 2004;198:42-50. [Crossref] [PubMed]
- Markar S, Gronnier C, Duhamel A, et al. The Impact of Severe Anastomotic Leak on Long-term Survival and Cancer Recurrence After Surgical Resection for Esophageal Malignancy. Ann Surg 2015;262:972-80. [Crossref] [PubMed]
- Turrentine FE, Denlinger CE, Simpson VB, et al. Morbidity, mortality, cost, and survival estimates of gastrointestinal anastomotic leaks. J Am Coll Surg 2015;220:195-206. [Crossref] [PubMed]
- Goense L, van Dijk WA, Govaert JA, et al. Hospital costs of complications after esophagectomy for cancer. Eur J Surg Oncol 2017;43:696-702. [Crossref] [PubMed]
- Moon SW, Kim JJ, Cho DG, et al. Early detection of complications: anastomotic leakage. J Thorac Dis 2019;11:S805-11. [Crossref] [PubMed]
- Messager M, Warlaumont M, Renaud F, et al. Recent improvements in the management of esophageal anastomotic leak after surgery for cancer. Eur J Surg Oncol 2017;43:258-69. [Crossref] [PubMed]
- Goense L, Stassen PM, Wessels FJ, et al. Diagnostic performance of a CT-based scoring system for diagnosis of anastomotic leakage after esophagectomy: comparison with subjective CT assessment. Eur Radiol 2017;27:4426-34. [Crossref] [PubMed]
- Power N, Atri M, Ryan S, et al. CT assessment of anastomotic bowel leak. Clin Radiol 2007;62:37-42. [Crossref] [PubMed]
- Kim TH, Kim JH, Shin CI, et al. CT findings suggesting anastomotic leak and predicting the recovery period following gastric surgery. Eur Radiol 2015;25:1958-66. [Crossref] [PubMed]
- Lewis I. The surgical treatment of carcinoma of the oesophagus; with special reference to a new operation for growths of the middle third. Br J Surg 1946;34:18-31. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806-8. [Crossref] [PubMed]
- Low DE, Alderson D, Cecconello I, et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015;262:286-94. [Crossref] [PubMed]
- Heinze G, Wallisch C, Dunkler D. Variable selection - A review and recommendations for the practicing statistician. Biom J 2018;60:431-49. [Crossref] [PubMed]
- Upponi S, Ganeshan A, D'Costa H, et al. Radiological detection of post-oesophagectomy anastomotic leak - a comparison between multidetector CT and fluoroscopy. Br J Radiol 2008;81:545-8. [Crossref] [PubMed]
- Strauss C, Mal F, Perniceni T, et al. Computed tomography versus water-soluble contrast swallow in the detection of intrathoracic anastomotic leak complicating esophagogastrectomy (Ivor Lewis): a prospective study in 97 patients. Ann Surg 2010;251:647-51. [Crossref] [PubMed]
- Hogan BA, Winter DC, Broe D, et al. Prospective trial comparing contrast swallow, computed tomography and endoscopy to identify anastomotic leak following oesophagogastric surgery. Surg Endosc 2008;22:767-71. [Crossref] [PubMed]
- Schaible A, Sauer P, Hartwig W, et al. Radiologic versus endoscopic evaluation of the conduit after esophageal resection: a prospective, blinded, intraindividually controlled diagnostic study. Surg Endosc 2014;28:2078-85. [Crossref] [PubMed]
- Aiolfi A, Asti E, Rausa E, et al. Use of C-reactive protein for the early prediction of anastomotic leak after esophagectomy: Systematic review and Bayesian meta-analysis. PLoS One 2018;13:e0209272. [Crossref] [PubMed]
- Straatman J, Cuesta MA, Schreurs WHH, et al. The PRECious trial PREdiction of Complications, a step-up approach, CRP first followed by CT-scan imaging to ensure quality control after major abdominal surgery: study protocol for a stepped-wedge trial. Trials 2015;16:382. [Crossref] [PubMed]