Prediction of clinical response to omalizumab in moderate-to-severe asthma patients using the change in total serum IgE level
Introduction
Immunoglobulin E (IgE) plays a central role in pathophysiological mechanisms of allergic reactions and bronchial airway inflammation (1-3). Based on this data, a recombinant humanized monoclonal anti-IgE antibody, omalizumab, was approved for the treatment of insufficiently controlled moderate-to-severe persistent IgE-mediated allergic asthma (4). Omalizumab targets the Fc region of free serum IgE, forms immune complexes, and reduces the serum level of free IgE (4). It also blocks allergic responses via the downregulation of the high affinity IgE receptor FcɛRI, which is present on inflammatory and structural cells, and by the inhibition of mast cell activation and the release of inflammatory factors by basophils (5-7). Omalizumab has been shown to reduce asthma-induced symptoms and exacerbations, and emergency department visits and hospitalizations (8-10). However, the Global Initiative for Asthma guidelines indicate that its cost is high, and that patient responses vary widely (4).
The dosage and frequency of omalizumab administration are calculated based on baseline total serum IgE level and bodyweight. However, baseline total serum IgE level does not dependably predict the response (11-13). It seems that free IgE, rather than total IgE, is closely related to response to omalizumab; however, it is difficult to monitor free serum IgE levels once omalizumab treatment is initiated. Furthermore, monitoring free IgE and omalizumab serum concentrations has not been shown to predict the clinical response or assist in deciding to continue or stop treatment (14).
Recently, baseline serum CXCL10 and interleukin (IL)-12 levels have been shown that they may predict severe asthmatics’ responsiveness to omalizumab; unfortunately, levels of these cytokines are low and blood levels cannot be measured reliably (12). Thus, there are no reliable predictors of the response to omalizumab in patients with asthma (12-15).
A recent study showed that monitoring the change of total serum IgE level in patients with chronic spontaneous urticaria can predict the response to omalizumab (16). The study showed that a ratio of total serum IgE level at 4 weeks after beginning treatment: baseline level ≥2 was associated with a high likelihood of adequate treatment response (16). Based on this finding, the purpose of this study was to determine if a change of total serum IgE level could predict the response to omalizumab in patients with moderate-to-severe asthma.
We present the following article in accordance with the IgE change predicts asthma patients’ response to omalizumab reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-2073).
Methods
Patients
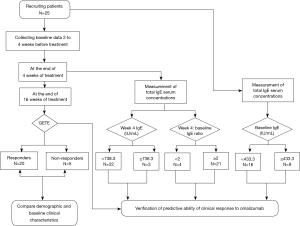
Twenty-five patients with uncontrolled moderate-to-severe persistent asthma were recruited from March 2018 to December 2019, from the respiratory outpatient clinic at Southern Medical University, Nanfang Hospital (Guangzhou, China) (Figure 1). Moderate-to-severe persistent uncontrolled asthma is characterized by frequent daily symptoms or nocturnal awakenings and exacerbations, despite a minimum of 3-step treatment (4,17). Moderate asthma was defined as under complete control using 3-step treatment. Severe asthma was defined as controlled or uncontrolled even using 4- or 5-step treatment (4,17). The diagnosis of asthma was based on physician observation of reversible airflow obstruction. Reversible airflow obstruction was defined as forced expiratory volume in 1 s (FEV1) increase from baseline ≥12% and FEV1 absolute value increase ≥200 mL after inhalation 400 µg salbutamol. All patients were treated with omalizumab based on national (17) and international guidelines (4). The total serum IgE levels and bodyweight of all patients were within the parameters described in the omalizumab dosing table.
Exclusion criteria were current smoking, serious comorbidities including bronchiectasis, ABPA and lung cancer, respiratory tract infection or acute exacerbation within the past 4 weeks, and the possibility of pregnancy. This study was approved by the Hospital Ethics Committee (code number NFEC-2018-092). All patients provided written informed consent for participation in the study.
Study design
This was an open single-center, prospective study of consecutive cases. Two to 4 weeks before beginning omalizumab treatment (baseline), patients were seen to confirm they were appropriate candidates for omalizumab treatment, their remission stage, and to collect demographic data (Figure 1). Baseline data collected included current medications, comorbidities, fractional exhaled nitric oxide (FeNO), blood eosinophilic count and ratio, eosinophilic ratio in induced sputum, and serum vitamin D level.
Twenty-three patients received monthly subcutaneous injections of omalizumab for a total of 16 weeks; two patients are injected every two weeks; the dosage was derived from the standard omalizumab dosing table. Patients received clinic visits prior to each injection, and at the end of the 16 weeks. Omalizumab effectiveness was evaluated after 16 weeks (4).
Total IgE serum concentrations were measured at baseline and at the end of week 4, prior to the second injection. Levels were measured using an electrochemical luminescence method (Roche, Germany), which is a commonly used method that measures both free and omalizumab-bound IgE. Serum samples were tested within 1 hour after collection. The ratio of week 4 IgE level:baseline IgE level was calculated.
Other data collected at baseline and each follow-up visit included: pre-bronchodilator lung function (according to ATS/ERS(American Thoracic Society/European Respiratory Society) criteria (18), Jaeger MasterScreen Body, Hoechberg, Germany), exhaled NO level according to ATS guidelines (Niox Mino, Aerocrine, Sweden), physician-assessed evaluation of global treatment effectiveness (GETE: excellent, good, moderate, poor, worsened) (19), and evaluation of asthma control with the asthma control questionnaire (ACQ) (20) and asthma control test (ACT) (21). The minimal clinically significant changes in ACQ and ACT scores are 0.5 and 3, respectively. Proper medication adjustment was permitted to improve a patient’s asthma control.
Statistical analysis
Patients rated as good or excellent by GETE were considered to be responders, and demographic and clinical characteristics were compared between responders and non-responders. The Kolmogorov Smirnov test was used to determine whether continuous variables were normally distributed. Levene’s test was used for the evaluation of homogeneity of variances. Continuous data (e.g., age) were presented as mean ± standard deviation. Non-normally distributed variables (e.g., total IgE) were presented as medians and interquartile ranges (IQR). Categorical data were presented as count and percentage. Continuous data were compared with the 2-sample t-test and categorical data with the Fisher’s exact test; non-normally distributed data were examined with the Mann Whitney U test.
Receiver operating characteristic (ROC) curve analyses were used to determine the predictive value of baseline IgE level, week 4 IgE level, and the week 4:baseline IgE ratio could predict response to omalizumab. The maximized likelihood was used to identify cutoff values, and to calculate the sensitivity and specificity. The area under the ROC curve (AUC) was calculated to evaluate the predictive ability of the variables examined. All analyses were performed using SPSS version 26.0 statistical software. A 2-tailed P value of <0.05 was regarded as statistically significant. Due to lack of distribution data of total serum IgE 4-week-to-baseline ratio levels in asthma patients after omalizumab treatment, power of test rather than intended sample size was calculated using PASS version 11.0 statistical software.
Results
Response to omalizumab treatment
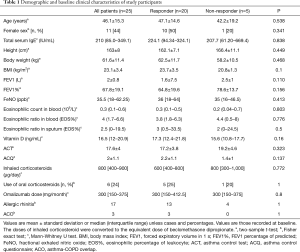
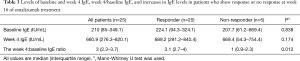
All 25 patients were treated with omalizumab for 16 weeks, and most patients had an effective response to treatment. After 16 weeks of treatment, 4 patients (16%) had an excellent response and 16 (64%) a good response; 5 patients (20%) showed no response. There were no significant differences in demographic and baseline clinical characteristics between responders and non-responders (Table 1). Lung function tended to be improved in responders. Oral corticosteroids could be withdrawn in 4 of the 5 responders who had been on oral corticosteroids before the study. Inhaled corticosteroid (ICS) doses were unchanged in all patients. The baseline ACT and ACQ scores in responders were 17.2±3.8 and 2.2±1.1, respectively. And the baseline ACT and ACQ scores in non-responders was 19.2±4.6 and 1.4±1, respectively. After 16 weeks of treatment, the average increase of ACT score was 3.7 and the average reduction of ACQ score was 1.4 in responders (both, P<0.01). In contrast, there was no clinically relevant improvement in the non-responders (Table 2).
Full table

Full table
Increases in IgE levels in responders and non-responders during the first 4 weeks
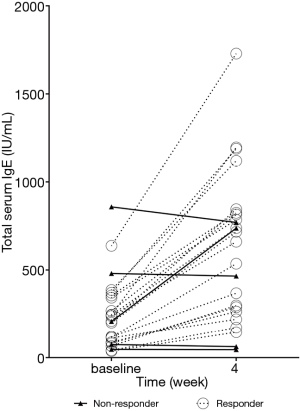
At week 4 after beginning treatment, responders exhibited a significant increase in total IgE levels; whereas there was no change in levels in non-responders (Table 2, Figure 2).
Predicting response to omalizumab treatment
There were no significant difference in baseline total serum IgE level and total serum IgE level at week 4 between responders and non-responders (Table 3).
Full table
However, the week 4 IgE level:baseline level ratio exhibited an AUC =0.87 (95% CI: 0.64–1) for predicting a response to omalizumab treatment (Figure 3). The best ratio cutoff value ranged from 1.6 to 2.2; thus, for subsequent analysis a cutoff value of 2 was used. Using a cutoff value of 2, the week 4 IgE level:baseline level ratio exhibited a sensitivity of 100% and specificity of 80% for predicting a response to omalizumab (Figure 3). With α=0.05, 21 patients’ week 4 IgE level:baseline level ratio over 2, 4 patients’ week 4 IgE level:baseline level ratio below 2, and an AUC of 0.87, the power of the test is 0.75.
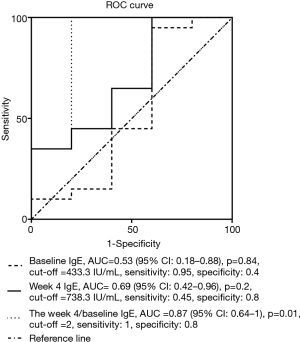
Twenty of 21 patients with a week 4 IgE level:baseline level ratio ≥2 were responders, while 4 of 4 patients with a ratio <2 were non-responders (at week 16).
Serum vitamin D level and other markers for predicting response to omalizumab
There were no differences in baseline FeNO, eosinophilic ratio in induced sputum, eosinophils in blood and blood eosinophil ratio, and serum vitamin D levels between responders and non-responders (Table 1).
Discussion
To our knowledge, this is the first study that has indicated that a more than 2-fold increase in the week 4 serum IgE:baseline IgE ratio can predict a good/excellent response to omalizumab in patients with moderate-to-severe asthma. Our findings proved once again that omalizumab can improve overall asthma control; at the end of 16 weeks of treatment 80% of patients benefited from omalizumab, which is similar to the results of another real-life study of 158 patients rated by GETE (22). More importantly, our results suggest a method to determine which patients will respond, i.e., the week 4: baseline IgE ratio may be useful for predicting patients who will respond to omalizumab within the first 16 weeks of treatment. Use of the ratio may help in deciding to continue or discontinue treatment.
During the first 4 weeks of treatment, the IgE levels of omalizumab responders increased, while that of non-responders did not change. The underlying mechanisms for these findings are not clear and further research is needed. One of the possible reasons could be that the free IgE of non-responder doesn’t combine with omalizumab. An anti-drug antibody (ADA) response, off-target binding, and glycosylation pattern can inhibit the binding of omalizumab and IgE (23). A lack of complex formation can result in faster clearance of IgE and no increase in levels and a poor therapeutic effect in non-responders. In addition, omalizumab–IgE complexes may not be inactive and can capture allergens, which may improve the therapeutic effects of omalizumab (24).
In a prospective study including 41 severe asthmatics, serum IgE levels were measured over 1 year at 7 time points, and the results showed that there was limited within-patient variability of IgE levels (25). This finding suggests that measurement of total IgE is a stable method to determine management. Also, in our study serum IgE levels were not measured during an exacerbation of asthma. However, during and after a severe exacerbation of asthma, serum IgE level may increase and may remain elevated for 1–2 months. Thus, total IgE levels should not be measured for 4–8 weeks after an exacerbation if they are going to be used to guide management (26).
In this study, we used GETE to measure treatment effectiveness. GETE is widely used for evaluating the response to omalizumab effectiveness (22,27). Most prior reports have evaluated responsiveness to omalizumab by monitoring a decrease of asthma exacerbations or an improvement in asthma quality of life questionnaire (AQLQ) score (15,28).
Our results showed that response to omalizumab cannot be predicted by blood eosinophil count, which is similar to the results of other studies (12,27).
GINA 2020 (4) and some other studies (12,15) have indicated that asthma patients with high FeNO levels are more likely to respond to omalizumab. Other studies, however, have arrived at opposite conclusions (28).
In this study, 3 patients with ACO (asthma-COPD overlap) had good treatment responses; this finding is consistent with that of a recent a large-scale, real-world study that indicated patients with ACO treated with omalizumab had similar clinical outcomes to patients with asthma without ACO in terms of improvements in exacerbation frequency and ACT score (29). Usually, high levels of IgE and FeNO, and a high blood eosinophil count are markers of a better response to omalizumab for ACO patients (30).
Vitamin D insufficiency is common in asthmatics, and low vitamin D levels are associated with asthma severity and higher odds of asthma exacerbation (31-33). Therefore, we also investigate the predictive value of vitamin D in treatment response of omalizumab. Our results showed that baseline Vitamin D levels cannot predict response to omalizumab.
Several limitations of this study need to be emphasized. First, the sample size was small; we plan to perform a future investigation with a larger number of patients. Second, we did not examine allergic responses with a specific serum IgE or skin prick test; however, some studies have shown that omalizumab can be effective in non-atopic severe asthma (34,35). Third, it cannot be excluded that the number of responders will increase with an increasing duration of treatment. However, it seems that the effectiveness after 16 weeks is similar to that of an extended course of treatment. A study of 258 patients demonstrated that the GETE at 16 weeks in omalizumab-treated asthma patients is an effective indicator of a persistent response, and could predict treatment response or nonresponse at week 32 in 83.3% of patients (36). Lastly, this study was not blinded and patients were aware of their total IgE levels.
Conclusions
The study showed that a week 4:baseline IgE ratio ≥2 predicts a good response to omalizumab at 16 weeks in patients with moderate-to-severe asthma. Future multi-center trials with a large sample size are required to verify the results.
Acknowledgments
The authors would like to thank the patients and others who cooperated in performing this study.
Funding: This work was supported by the Precision Medicine Research of The National Key Research and Development Plan of China (2016YFC0905800), the National Natural Science Foundation of China (81770033, 81670026, 81700034, 81870026), the Scientific and Technological Project of Guangdong Province (2016A020215117, 2017B020226006, 201804010069) and the Science and Technology Program of Guangzhou, China (201804010069).
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-2073
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-2073). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Nanfang Hospital, Southern Medical University (code number NFEC-2018-092). All adult subjects and the parents of children patients have given their written informed consent prior to participation in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol 2008;8:205-17. [Crossref] [PubMed]
- Balbino B, Conde E, Marichal T, et al. Approaches to target IgE antibodies in allergic diseases. Pharmacol Ther 2018;191:50-64. [Crossref] [PubMed]
- Chung KF. Anti-IgE monoclonal antibody, omalizumab: a new treatment for allergic asthma. Expert Opin Pharmacother 2004;5:439-46. [Crossref] [PubMed]
- Global strategy for asthma management and prevention: Updated 2020. Available online: http://ginasthma.org
- Liu J, Lester P, Builder S, et al. Characterization of complex formation by humanized anti-IgE monoclonal antibody and monoclonal human IgE. Biochemistry 1995;34:10474-82. [Crossref] [PubMed]
- Chapman KR, Cartier A, Hébert J, et al. The role of omalizumab in the treatment of severe allergic asthma. Can Respir J 2006;13 Suppl B:1B-9B.
- MacGlashan DW Jr, Bochner BS, Adelman DC, et al. Down-regulation of Fc(epsilon)RI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol 1997;158:1438-45. [PubMed]
- Holgate S, Buhl R, Bousquet J, et al. The use of omalizumab in the treatment of severe allergic asthma: A clinical experience update. Respir Med 2009;103:1098-113. [Crossref] [PubMed]
- Bousquet J, Cabrera P, Berkman N, et al. The effect of treatment with omalizumab, an anti-IgE antibody, on asthma exacerbations and emergency medical visits in patients with severe persistent asthma. Allergy 2005;60:302-8. [Crossref] [PubMed]
- Korn S, Thielen A, Seyfried S, et al. Omalizumab in patients with severe persistent allergic asthma in a real-life setting in Germany. Respir Med 2009;103:1725-31. [Crossref] [PubMed]
- Lanier BQ. Unanswered questions and warnings involving anti-immunoglobulin E therapy based on 2-year observation of clinical experience. Allergy Asthma Proc 2005;26:435-9. [PubMed]
- Suzukawa M, Matsumoto H, Ohshima N, et al. Baseline serum CXCL10 and IL-12 levels may predict severe asthmatics' responsiveness to omalizumab. Respir Med 2018;134:95-102. [Crossref] [PubMed]
- Bousquet J, Wenzel S, Holgate S, et al. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest 2004;125:1378-86. [Crossref] [PubMed]
- Korn S, Haasler I, Fliedner F, et al. Monitoring free serum IgE in severe asthma patients treated with omalizumab. Respir Med 2012;106:1494-500. [Crossref] [PubMed]
- Hanania NA, Wenzel S, Rosen K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med 2013;187:804-11. [Crossref] [PubMed]
- Ertas R, Ozyurt K, Atasoy M, et al. The clinical response to omalizumab in chronic spontaneous urticaria patients is linked to and predicted by IgE levels and their change. Allergy 2018;73:705-12. [Crossref] [PubMed]
- Expert Group of Omalizumab for Allergic Asthma CMARBAG. Chinese expert consensus on omazumab for allergic asthma. Chinese Journal of Tuberculosis and Respiratory Diseases 2018;41:179-85.
- Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014;43:343-73. [Crossref] [PubMed]
- Price D. The use of omalizumab in asthma. Prim Care Respir J 2008;17:62-72. [Crossref] [PubMed]
- Juniper EF, O'Byrne PM, Guyatt GH, et al. Development and validation of a questionnaire to measure asthma control. Eur Respir J 1999;14:902-7. [Crossref] [PubMed]
- Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol 2004;113:59-65. [Crossref] [PubMed]
- Brusselle G, Michils A, Louis R, et al. "Real-life" effectiveness of omalizumab in patients with severe persistent allergic asthma: The PERSIST study. Respir Med 2009;103:1633-42. [Crossref] [PubMed]
- Matera MG, Calzetta L, Rogliani P, et al. Monoclonal antibodies for severe asthma: Pharmacokinetic profiles. Respir Med 2019;153:3-13. [Crossref] [PubMed]
- Hsu CL, Shiung YY, Lin BL, et al. Accumulated immune complexes of IgE and omalizumab trap allergens in an in vitro model. Int Immunopharmacol 2010;10:533-9. [Crossref] [PubMed]
- Louis R, Pilette C, Michel O, et al. Variability in total serum IgE over 1 year in severe asthmatics. Allergy Asthma Clin Immunol 2019;15:20. [Crossref] [PubMed]
- Semprini R, Shortt N, Ebmeier S, et al. Change in biomarkers of type-2 inflammation following severe exacerbations of asthma. Thorax 2019;74:95-8. [Crossref] [PubMed]
- Bousquet J, Rabe K, Humbert M, et al. Predicting and evaluating response to omalizumab in patients with severe allergic asthma. Respir Med 2007;101:1483-92. [Crossref] [PubMed]
- Busse W, Corren J, Lanier BQ, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol 2001;108:184-90. [Crossref] [PubMed]
- Hanania NA, Chipps BE, Griffin NM, et al. Omalizumab effectiveness in asthma-COPD overlap: Post hoc analysis of PROSPERO. J Allergy Clin Immunol 2019;143:1629-33.e2. [Crossref] [PubMed]
- Sposato B, Scalese M, Milanese M, et al. Should omalizumab be used in severe asthma/COPD overlap? J Biol Regul Homeost Agents 2018;32:755-61. [PubMed]
- de Groot JC, van Roon EN, Storm H, et al. Vitamin D reduces eosinophilic airway inflammation in nonatopic asthma. J Allergy Clin Immunol 2015;135:670-5.e3. [Crossref] [PubMed]
- Korn S, Hübner M, Jung M, Blettner M, et al. Severe and uncontrolled adult asthma is associated with vitamin D insufficiency and deficiency. Respir Res 2013;14:25. [Crossref] [PubMed]
- Salas NM, Luo L, Harkins MS, et al. Vitamin D deficiency and adult asthma exacerbations. J Asthma. 2014;51:950-5. [Crossref] [PubMed]
- de Llano LP, Vennera MC, Alvarez FJ, et al. Effects of omalizumab in non-atopic asthma: results from a Spanish multicenter registry. J Asthma 2013;50:296-301. [Crossref] [PubMed]
- Çelebi Sözener Z, Aydın Ö, Mısırlıgil Z, et al. Omalizumab in non-allergic Asthma: A report of 13 cases. J Asthma 2018;55:756-63. [Crossref] [PubMed]
- Bousquet J, Siergiejko Z, Świebocka E, et al. Persistency of response to omalizumab therapy in severe allergic (IgE-mediated) asthma. Allergy 2011;66:671-8. [Crossref] [PubMed]