International consensus statement on robot-assisted minimally invasive esophagectomy (RAMIE)
Introduction
The technique of minimally invasive esophagectomy (MIE) is constantly evolving. According to an international survey, the proportion of surgeons performing MIE has increased from only 14% in 2009 to more than 50% in 2014 (1,2). MIE has been proven to improve perioperative results by MORO and TIME trials (3-6), and with comparable disease-free survival (DFS) and overall survival (OS) to open surgery. However, thoracoscopic-assisted MIE requires a long learning curve and accumulation of a large number of cases. Both large Asian medical centers and a national database review in the United States indicated that the reoperation rate of MIE was significantly higher than that of open surgery during the same period (7,8). In the process of developing thoracoscopic procedure, it is possible to sacrifice the principles of oncology, especially in the learning stage.
The emergence of robotic technology may overcome some of the shortcomings of traditional MIE, enabling junior esophageal and open surgeons to quickly complete minimally invasive transformation. Robot-assisted minimally invasive esophagectomy (RAMIE) was first performed by Dr. Santi Horgan in August 2002 using a transhiatal approach. RAMIE-McKeown appeared in 2002 (9), and a series of case reports was formed in 2006 (10). Since then, centers in Europe, North America and Asia have reported their own experiences of RAMIE. Based on the literature to date, the feasibility and safety of RAMIE appears to be similar to MIE and open esophagectomy (11-14). The ROBOT trial is presently the only randomized controlled trial (RCT) to compare RAMIE and open esophagectomy. Results of the ROBOT trial have indicated better perioperative recovery with RAMIE and comparable oncological outcomes (15). However, no previous RCT study demonstrated the superiority of RAMIE to traditional thoracoscopy, with the exception of two ongoing studies of REVATE and RAMIE (16,17). Retrospective studies suggest improved lymphadenectomy, decreased intraoperative blood loss, and potentially shorter intensive care unit stays (18).
At present, the RAMIE technique is only routinely carried out in a few large centers. As a new technology, consensus statements can shorten the learning curve for practitioners and provide specific guidance for quickly mastering the technology. The Upper GI International Robotic Association has focused on similar work before, as The Da Vinci Ivor Lewis Esophagectomy Procedure Guide was described in detail in their consensus. However, the limitation of this consensus is that it does not fully consider the surgical principle and technical habits of robotic surgery for esophageal squamous cell carcinoma (ESCC), especially Asian ESCC, such as radical dissection of upper mediastinal lymph nodes and the three-incision surgical approach (19). This portion of patients accounts for more than half of the world’s esophageal cancer patients.
To summarize technical experience with RAMIE on a larger scale, shorten the learning curve for the beginners, improve the safety of surgery, and judge the feasibility of RAMIE technology through the participation of more surgeons, we invited representative international experts to participate in the development of this consensus. In addition, we searched online databases for published articles related to RAMIE; for evidence-based methods, all evidences were graded using the GRADE system and upgraded or downgraded after integrating experts’ opinions until a final consensus statement was reached. We present the following article in accordance with the AGREE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-1945).
Methods
We referred to the World Health Organization Handbook for Guideline Development and established the Consensus Development Group (CDG). We identified potential experts by performing a literature review using the PubMed/Medline database with the keywords ‘‘robot’’ and ‘‘esophagectomy’’. Experts who agreed to participate were also requested to invite respective regional experts in robotic esophagectomy. An initial working group identified representative international esophageal surgical centers and individual surgeons. Experts were defined as having performed more than 50 cases of robotic esophagectomy, as we previously demonstrated a significant reduction of operating time after 40 cases (18). A total of 23 international experts were initially identified, of which 21 agreed to participate in the consensus statements, with the following missions:
- Define the scope of the consensus and draft key questions relevant to the current status of RAMIE;
- Administer questionnaires to experts and tabulate the results;
- Grade the quality of the evidence;
- Draft preliminary recommendations;
- Write the draft consensus;
- Publish and promote consensus statements.
Experts in the CDG voted on the recommendations according to the quality of evidences and surgeons’ preferences. The GRADE Grid method and Delphi vote were used to formulate the recommendations. The GRADE grid allows experts on the consensus panel to record their views about the balance between advantages and disadvantages of a specific procedure, based on their opinion of the available evidence. To guide the use of GRADE grid, all participants received instructions describing factors that influence the strength of a recommendation and the implications of strong and weak recommendations.
We defined the way of assigning levels of evidences and statements as follows.
- Factors that influence the strength of a recommendation
- Balance between desirable and undesirable effects—the larger the difference between desirable and undesirable effects, the more likely a strong recommendation is warranted. The narrower the gradient, the more likely a weak recommendation is warranted.
- Quality of evidence—the higher the quality of evidence, the more likely a strong recommendation is warranted.
- Values and preferences—the more variability in values and preferences, or more uncertainty in values and preferences, the more likely a weak recommendation is warranted.
- Implications of strong and weak recommendations
- Strong recommendation
Most patients in this situation would benefit from the recommended course of action, while only a small proportion would not. Most surgeons should agree with the consensus statement. - Weak recommendation
Most patients in this situation would not benefit from the course of action, which may even be harmful to health. Most surgeons would not agree with the consensus statement. - No specific recommendation
If the advantages and disadvantages of the referred consensus statement are equivalent, or there is not sufficient evidence to support the recommendation.
In May 2019, all experts were invited by e-mail to participate in the consensus statement and received a personal Excel file to fill out the survey. Two rounds of voting were conducted. It was decided by the group that when 66.7% of the experts approved the recommendation, a consensus had been reached and the statement could be made. To provide methodological support, the Consensus Secretary Group (CSG) performed a systematic review of the literature and investigation of surgeons’ preferences, along with the Chinese GRADE Center. All members of the CDG and CSG were required to disclose potential conflicts of interest, which were reviewed by the group chairs.
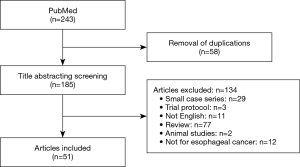
For literature review (Figure 1), the PubMed database was searched for: “robot” AND “esophagectomy”, and their synonyms or abbreviations. No additional search software or special features were used. The search was limited to papers describing original patient data for a series of more than 10 patients in the English language. The investigators (BL and YY) independently performed screening and article selection procedures. All articles that fulfilled the eligibility criteria were included in our systematic review. Duplicate publications with derivative patients were excluded. Search results and the selection process were summarized in a flowchart. Each article was screened for first author, year of publication, number of subjects, study population characteristics, and study design. Furthermore, postoperative complications, and oncologic results were assessed.
Results
Twenty-three international surgeons who are experts in the field of robotic esophagectomy were invited to participate in the consensus statement. As mentioned above, two rounds of electronic surveys were carried out. A total of 8 topics and 91 questions were reviewed and voted on by 21 experts (two experts did not provide responses in the first round). After applying the selection criteria to all searched abstracts, 51 articles remained available for analysis. Finally, 27 statements were recommended based on the experts’ opinions and systematic literature review. The main topics of the statements fall under the following areas: surgical approach, anesthesia, patient position, trocar arrangement, surgical technique, learning curve, complications, and survival outcomes.
Surgical approach
- RAMIE is always the preferred approach when the robot machine is available, regardless of clinical stage or whether neoadjuvant therapy has been received.
GRADE of evidence: low; strength of recommendation: weak. Consensus reached: 78% agreement. - The transthoracic esophagectomy (TTE) is the preferred approach in RAMIE, especially for squamous cell carcinoma.
GRADE of evidence: low; strength of recommendation: strong.
Consensus reached: 67% agreement. - Transhiatal esophagectomy (THE) is safe and feasible for adenocarcinoma.
GRADE of evidence: low; strength of recommendation: weak.
Consensus reached: 78% agreement.
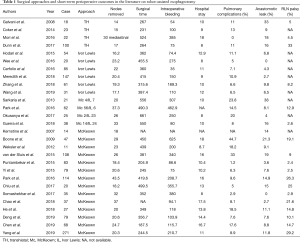
RAMIE can be performed by two main approaches: transhiatal and transthoracic. The choice of surgical approach for RAMIE is based on the surgeon’s experience and characteristics of the tumor. We analyzed data from 27 centers that had performed RAMIE for more than 10 cases. Among them, the transhiatal approach was adopted in 4 centers (14.8%), the Ivor Lewis procedure in 5 centers (18.5%), and the McKeown procedure in 13 centers (48.1%); both Ivor Lewis and McKeown procedures were performed in the remaining 5 centers (18.5%) (Table 1).
Full table
TTE
In Asia, most surgeons prefer the RAMIE McKeown procedure (three incisions). This is due in large part to the greater prevalence of squamous cell carcinoma in the patient population and, accordingly, an increased need to manage the biology of squamous cell cancer (20,21). RAMIE-Ivor Lewis appears to be the preference for middle and lower esophageal cancer (22-24). Again, this procedure addresses the biology of the disease and appears to have a lower rate of anastomotic leak. Based on concerns about anastomotic leakage, an increasing number of European and American experts have turned to Ivor Lewis technology. According to our analysis (Table 1), the Ivor Lewis procedure was applied in 10 (43.5%) of the 23 centers, of which 3 centers (30%) were in Asia; whereas, the McKeown procedure was performed in 18 centers (78.3%), of which 11 centers (61.1%) were in Asia. Emphasis on the McKeown technique in Asia stems from deep attention to upper mediastinum and cervical lymph node dissection, as well as a high incidence of middle and upper thoracic squamous cell carcinomas. In addition, the incidence of cervical anastomotic leakage in Asian patients has always remained within an acceptable range.
THE
THE, which is thought to preserve pulmonary function and potentially enhance postoperative recovery (25,26), is frequently applied for the treatment of adenocarcinoma of the lower esophagus or early squamous cell esophageal cancer (27,28). THE was the first robotic-assisted esophagectomy performed, mainly because robotic surgery at the time had only been carried out on the abdomen, and so it started with this hybrid approach (29). At present, robotic dissection can reach freely up to the lower pulmonary vein through the hiatus, but cannot easily reach the carina. Moreover, whether THE allows radical mediastinal lymphadenectomy remains a concern, which is the key difference from a transthoracic procedure. Increased likelihood of mediastinal bleeding and airway damage also remain important issues for THE. To further improve the efficiency of lymphadenectomy during THE, Mori et al. (30) developed a non-transthoracic esophagectomy procedure combining RATHE and a video-assisted cervical approach. The authors believed that this procedure could achieve the same lymph node dissection result as the transthoracic approach while significantly reducing postoperative pulmonary complications. These techniques provide a new direction for RATHE; however, studies with larger sample sizes are still required to verify their safety and feasibility. In the future, robots may also be used in transhiatal esophageal surgery. One key technique that still needs to be addressed is a flexible, single-arm, multi-operation tunnel technology, such as the SP system. A Japanese surgeon has also developed a non-transthoracic esophagectomy procedure comprising RATHE and a video-assisted cervical approach, but it is still far from a full robotic non-transthoracic esophagectomy (30,31).
Anesthesia and patient’s position
- CO2 insufflation is routinely applied during the thoracic phase of RAMIE-McKeown.
GRADE of evidence: low; strength of recommendation: strong.
Consensus reached: 89% agreement. - Proper trocar placement and the patient’s position are crucial for RAMIE.
GRADE of evidence: low; strength of recommendation: strong.
Consensus reached: 100% agreement. - A third robotic arm helps to achieve better surgical exposure.
GRADE of evidence: low; strength of recommendation: weak.
Consensus reached: 78% agreement.
Anesthesia management and patient positioning during RAMIE are very similar to those of MIE. Single-lumen endotracheal intubation with a right bronchial blocker and CO2 insufflation assistance is the most common procedure for thoracic exposure in Asia. CO2 insufflation can expand the mediastinum and maximize exposure of the intrathoracic esophagus by means of depressing the diaphragm caudally and pushing the lung anteriorly (32). Single-lumen tracheal intubation can facilitate lymph node dissection at the carina and upper mediastinum, especially 106recL nodes along the left of the recurrent laryngeal nerve (RLN). Double-lumen tracheal intubation is preferred by European and American doctors, either for Ivor Lewis or McKeown approaches, and is reliable to provide a safe surgical environment.
Semi-prone position (30–45 degree) is used for the thoracic phase of RAMIE by both Asian and Europe surgeons for McKeown esophagectomy, while the left lateral decubitus position is commonly used for Ivor Lewis (33-37). Trugeda et al. (38) reported utilization of the prone position for the RAMIE-Ivor Lewis procedure because they believed that this position allowed for better exposure of the esophagus and less injury to the lungs and hilar structures. However, this method is rarely adopted by other surgeons (39). Reverse Trendelenburg position is the basic position for upper gastrointestinal dissection, as well as the abdominal phase of RAMIE.
Trocar arrangement depends on the surgeon’s preference. Proper trocar placement is strongly recommended in the consensus statement, but we have not given a recommended uniform method. As every doctor has their own preferences and tendencies, trocar placement will be different. Even so, proper trocar placement is the basic requirement for a successful RAMIE, which has been agreed upon by all experts. According to the surgeon’s previous experience, three or four robotic arms are commonly applied for RAMIE. For RAMIE-Ivor Lewis, the four-arm mode is recommended because it helps to complete the thoracic anastomosis (40). For RAMIE-McKeown, use of the three-arm mode with the help of an assistant can effectively complete dissection of the thoracic esophagus and mediastinal lymph nodes (41). Use of the third robot arm (controlled by the operator) allows excellent operating exposure to be achieved through the application of stable and self-controllable tractions and counteractions on the esophagus and trachea. With the help of a third robotic arm, the surgeon can perform left RLN LND while maintaining full use of the primary ‘‘working’’ arms, ultimately ensuring a safer and easier approach to nodal dissection (20).
En bloc resection, total mesoesophagus excision (TME), and thoracic duct resection
- TME is recommended in RAMIE because it may result in better local control of tumor recurrence.
GRADE of evidence: low; strength of recommendation: weak.
Consensus reached: 67% agreement. - En bloc resection of tumors and lymph nodes is mandatory; however, the thoracic duct is not routinely resected.
GRADE of evidence: low; strength of recommendation: weak.
Consensus reached: 78% agreement. - Prophylactic ligation of the thoracic duct cannot prevent postoperative chylothorax.
GRADE of evidence: low; strength of recommendation: weak.
Consensus reached: 78% agreement.
The concept of TME comes from radical surgery for rectal cancer (42) and has been carried forward by Japanese surgeons for esophageal cancer resection. The essence of TME for the treatment of esophageal cancer is to use the anatomical space between the esophagus and its adjacent structures to maximally excise the esophagus and nerves, blood vessels, and lymphatic adipose tissues around it, which emphasizes the importance of en bloc excision of the mesoesophagus. However, some studies indicated that there was no difference between TME and non-TME with regard to the number of lymph nodes harvested. Nevertheless, Akiyama et al. (43) showed that the overall recurrence rate was significantly lower in the TME group (23.0% vs. 43.4%; P=0.011). Specifically, TME resulted in a decreased rate of mediastinal lymph node recurrence (2.3% vs. 11.3%; P=0.026). Thus, TME may have an advantage for local control of tumor recurrence. But this is a purely Asian concept, and it is very difficult to accurately identify the mesoesophagus, especially in patients who have undergone neoadjuvant radiotherapy. But with the assistance of robotic technology, TME can more easily be performed.
As it has been shown that the thoracic duct can contain metastatic tumor cells (44,45), it is recommended to remove the thoracic duct and its surrounding tissue upon the completion of TME (43,46). Schurink et al. (44) also demonstrated the presence of thoracic duct lymph nodes in 86% of cadaveric specimens, implying the possibility of node metastasis. However, thoracic duct resection may involve many physiological changes, such as the risk of postoperative chylothorax, changes in long-term immune status, possible hemodynamic effects, and absorption of nutrients from abdominal lymphatic drainage (47). Indeed, some studies demonstrated that thoracic duct resection is associated with poor prognosis and regional lymph node relapse in ESCC (48,49). Therefore, routine use of thoracic duct resection remains controversial (43,46). In terms of surgical techniques, RAMIE can more easily complete thoracic duct resection (47,50).
Anastomosis
- The mechanical stapler technique is recommended for anastomosis in RAMIE.
GRADE of evidence: low; strength of recommendation: weak.
Consensus reached: 78% agreement. - Intrathoracic anastomosis should be made as high as possible, and may be more accessible in RAMIE compared with MIE.
GRADE of evidence: low; strength of recommendation: weak.
Consensus reached: 67% agreement. - Reinforcement of the staple line of the gastric conduit is not routinely advised due to a lack of high-level evidence.
GRADE of evidence: low; strength of recommendation: weak.
Consensus reached: 67% agreement.
It is well known that a higher position of intrathoracic anastomosis can ensure a better resection margin and reduce the symptoms of reflux, especially for ESCC patients in Asia. The technical specifications for anastomosis were listed as follows: (I) anastomotic configuration: end-to-end, end-to-side, side-to-end, or side-to-side; (II) technique: robotic hand-sewn, circular stapling, or linear stapling; (III) suture technique: single layer, double layer, continuous, or interrupted; (IV) reinforcement of the anastomosis with omentum or pleura. Conventional thoracoscopic anastomosis techniques can be performed better using the robotic technology.
Hand-sewn intrathoracic anastomosis can reduce the incidence of postoperative anastomotic stenosis; however, due to its high technical demands, few surgeons attempt this technique under conventional thoracoscopy. Robotic assistance makes this technology a reality with a minimally invasive approach. The “T” shape or over-flap anastomosis with linear stapling technique has been used by many surgeons for anastomosis orifices with a wide diameter. The circular stapler remains the most reliable instrument to assist with an anastomosis, although it carries the disadvantage of complications associated with stricture (51). To reduce the likelihood for anastomotic leak, an omentum flap can be performed to reinforce Ivor Lewis anastomosis.
Several meta-analyses have compared stapled and hand-sewn anastomotic techniques. These studies have included both randomized and non-randomized trials, and have found no significant differences in anastomotic leak rates between the two anastomotic techniques (52-54). A network meta-analysis conducted by Kamarajah (55) demonstrated that stapled anastomosis, including circular- and linear-stapled, are associated with lower anastomotic leak rates compared with hand-sewn anastomosis following esophagectomy. Moreover, linear-stapled anastomosis is associated with a lower rate of anastomotic stricture compared with hand-sewn anastomosis. In the future, large high-quality randomized trial data are needed to provide evidence to compare the three anastomosis techniques.
Lymph node dissection
- RAMIE should be superior to MIE in terms of upper mediastinal lymph node dissection.
GRADE of evidence: moderate; strength of recommendation: strong.
Consensus reached: 89% agreement. - Lymph node dissection along the bilateral RLNs is recommended; however, skeletonization of RLNs is not widely performed.
GRADE of evidence: moderate; strength of recommendation: weak.
Consensus reached: 67% agreement. - Skeletonization of the celiac axis is recommended to achieve radical node dissection.
GRADE of evidence: low; strength of recommendation: weak.
Consensus reached: 67% agreement. - Cervical para-esophageal lymph nodes (No. 101) can be partially dissected during the thoracic phase of RAMIE.
GRADE of evidence: low; strength of recommendation: weak.
Consensus reached: 67% agreement. - Subcarinal lymph nodes must be routinely dissected.
GRADE of evidence: low; strength of recommendation: strong.
Consensus reached: 100% agreement.
Lymphadenectomy is a critical component of radical esophagectomy for the treatment of esophageal cancer. Therefore, the majority of studies concerning the application of RAMIE have focused on better lymphadenectomy, especially for mediastinal lymph node dissection. However, the conclusions derived from previous studies are not consistent. Retrospective studies of Ivor Lewis and McKeown procedures by Park et al. (22) and Deng et al. (56) found that RAMIE yielded more dissected lymph nodes than conventional MIE. However, several other studies have demonstrated that the number of total dissected lymph nodes was comparable between the two surgical techniques (57,58). van Rijswijk and colleagues (59) conducted an international survey to identify differences in esophageal cancer surgery in terms of surgical approach and extent of lymphadenectomy. In their survey, the extent of lymphadenectomy showed great variation among all experts. Users of the Japanese Society of Esophageal Diseases (JSED) classification (used by 100% of Asian respondents) performed a more extended cervical lymphadenectomy, as opposed to users of the American Joint Committee on Cancer classification (used by 79% of European respondents) (60). Therefore, it is difficult for us to infer which surgery is more conducive to lymph node dissection by means of comparing the results of different regions. Thus, clinical research with RCTs should be used to answer this question.
Based on their rich experience of RAMIE and MIE, 89% of experts in this consensus agreed on the comment “RAMIE should be superior to MIE in terms of extensive mediastinal lymphadenectomy”. The advantage of robotic surgery lies in its fine operation in a narrow space, which has been proven by its utilization for the dissection of upper mediastinal lymph nodes of ESCC. In Asia, RAMIE has been found to be more consistently thorough for mediastinal lymphadenectomy compared with MIE. The mediastinum, especially along both RLNs, is a common site for early metastasis and spread of upper and middle ESCC (61,62). Kim et al. (63) demonstrated that the skeletonization of RLNs results in an increased number of mediastinal lymph nodes harvested (30.3 vs. 19.6) and lymph nodes along RLN chains (13.5 vs. 4.8). Although RLN palsy was more common in patients with skeletonization of RLNs (31.8 vs. 5.6), all of these patients recovered within 1 year after surgery. In another propensity-matched study, Chao et al. (20) reported that skeletonization of RLNs did not increase the efficacy of lymph node dissection, as well as a similar incidence of RLN palsy. Lymph nodes at stations 16a1 and 16a2 of the para-aortic, truncus coeliacus, posterior surface of the pancreatic head, and arteria hepatica communis were the primary sites for abdominal metastasis after radical surgery for esophageal cancer (64). During the abdominal phase, skeletonization of the celiac axis is always recommended to achieve radical lymph node dissection, either for adenocarcinoma or squamous cell carcinoma.
Perioperative outcomes
- RAMIE does not increase the incidence of intraoperative adverse events compared with open or thoracoscopic approaches.
GRADE of evidence: moderate; strength of recommendation: strong.
Consensus reached: 83% agreement. - Intraoperative airway injury can be repaired with robotic assistance.
GRADE of evidence: low; strength of recommendation: strong.
Consensus reached: 100% agreement. - The overall rate of postoperative complications in RAMIE is comparable with MIE, while the incidence of RLN injury is lower than MIE.
GRADE of evidence: moderate; strength of recommendation: weak.
Consensus reached: 67% agreement. - RAMIE can decrease the incidence of postoperative pulmonary complications compared with open esophagectomy. GRADE of evidence: moderate; strength of recommendation: strong. Consensus reached: 89% agreement.
Unplanned events of RAMIE include but are not limited to the following: thoracic or abdominal adhesions, trocar puncture-related iatrogenic injury, RLN injury, airway injury, intraoperative hemorrhage, intraoperative ventricular arrhythmia, and R2 resection. The occurrence of intraoperative unplanned events may increase the incidence of postoperative complications and compromise the quality of tumor resection. If an unexpected event occurs during the operation, RAMIE is better equipped to fix it compared with traditional thoracoscopy, such as adhesion dissection, airway injury repair, and R0 resection of the invading tumor.
Incidence of postoperative complications after RAMIE differs among different centers, depending on the histologic type, surgical procedure, reconstruction approach, and lymph node dissection (Table 1). Generally, RAMIE did not change the 90-day mortality rate compared with MIE. For the McKeown procedure, the incidence of postoperative pulmonary complications was 7.6–18.5%, the incidence of anastomotic leakage was 2.7–14.9%, the incidence of vocal cord paralysis (VCP) was 10.1–26.3%, the length of postoperative hospital stay was 8–17 days, and the postoperative 90-day mortality rate was 0–2.7% (65,66). However, results reported in the literature are primarily from single-center retrospective studies, which do not reflect real-world postoperative complications after RAMIE. The ROBOT trial demonstrated the minimally invasive effect of RAMIE, as well as better perioperative recovery and comparable oncological outcomes compared with open esophagectomy for the treatment of esophageal cancer (15).
With regard to quality of life (QOL) after RAMIE, several groups have reported outcomes. Both van der Sluis et al. (15) and Sarkaria et al. (67) demonstrated that RAMIE is safe and results in reduced perioperative morbidity, improved early QOL, and equivalent oncological outcomes compared with open esophagectomy.
Thus far, no related study has focused on anastomotic stricture after RAMIE. Anastomotic stricture with dysphagia has a negative effect on the patient’s QOL and considerably worsens the patient’s postoperative nutritional status. Most strictures develop within the first 2–3 months after operation, and up to 50% are minimal, as assessed by endoscopy (68). Improvement of anastomotic techniques and application of the circular stapler have effectively reduced leak rates following esophagogastrostomy, but stricture formation has become more frequent because of stapler size limitations and anastomotic scar formation (69). Chen and co-workers reported that semi-mechanical esophagogastric anastomosis could prevent stricture formation more effectively than hand-sewn or circular stapler esophagogastrostomy, without increasing gastroesophageal reflux (70). According to the literature, the main potential risk factors associated with postoperative benign anastomotic stricture include anastomotic technique, limited circular stapler diameter, poor vascular supply, and anastomotic leak, which significantly impair long-term QOL (71,72).
Learning curve
- Operative time is an essential index of the learning curve for RAMIE.
GRADE of evidence: moderate; strength of recommendation: weak.
Consensus reached: 78% agreement. - The efficacy of lymphadenectomy is an essential index of the learning curve for RAMIE.
GRADE of evidence: moderate; strength of recommendation: strong.
Consensus reached: 94% agreement. - The learning curve of RAMIE is shorter than MIE in the thoracic phase.
GRADE of evidence: low; strength of recommendation: weak.
Consensus reached: 78% agreement.
Few reports describe the learning curve for RAMIE. Central evaluation indicators for the learning curve include docking time, operative time, efficacy of lymphadenectomy, postoperative complications, and postoperative stay. Regarding the operation time, there was no agreement in our questionnaire. In previous studies comparing RAMIE and MIE, the operation time was also inconsistent (58,73). In our opinion, use of robots should improve the efficiency of esophagectomy in RAMIE after achieving proficient docking and undocking. However, future RCT including the RAMIE trial (18) will give us the answers. Several previous studies reported that the learning curve for surgeons with experience in RAMIE is about 20 to 40 cases, which is significantly shorter than for MIE, especially for the thoracic phase (74,75). Park et al. (76) indicated that a minimum of 20 cases is required before a surgeon is experienced enough to perform safe dissection of bilateral RLN nodes. In another retrospective study (73), the authors reported that the most accurate evaluation indicators of the learning curve for RAMIE were operative time, lymph node dissection, complications, and length of hospital stay. van der Sluis et al. (77) demonstrated that a structured proctoring program could shorten the learning curve for young surgeons. Zhang et al. (78) found that surgeons with extensive experience in open and laparoscopic surgeries for esophageal cancer could reach proficiency in the RAMIE McKeown procedure after 26 cases. This was also the first study to describe the learning curve of assistants involved in RAMIE, especially for docking and undocking time. Evaluation of the learning curve for RAMIE in newly introduced surgeons should be divided into two levels: first, the surgical completion rate is evaluated according to the average operative time; second, the quality of surgery is evaluated according to the learning curve judgment indicators. Lymphadenectomy, which is a critical component of radical esophagectomy in the treatment of esophageal cancer, should be considered an essential index to assess the learning curve for RAMIE.
Survival outcomes
- RAMIE can achieve better local control of tumor recurrence compared with MIE.
GRADE of evidence: low; strength of recommendation: weak.
Consensus reached: 67% agreement. - Long-term survival outcomes of RAMIE compared with MIE and open esophagectomy still need further evidence.
GRADE of evidence: moderate; strength of recommendation: strong.
Consensus reached: 100% agreement. - Improvement in long-term survival will promote the development of RAMIE.
GRADE of evidence: low; strength of recommendation: weak.
Consensus reached: 72% agreement.
As a relatively recent technology, data regarding the oncologic efficacy and long-term survival of RAMIE are limited. As such, ongoing and further RCTs are called for to show improved outcomes of RAMIE. We believe that improvement in long-term survival will promote the development of RAMIE.
For ESCC treatment, RAMIE should be superior to MIE in terms of extensive mediastinal lymphadenectomy. Although no high-quality literature supports this statement at present, several previous retrospective studies demonstrated that RAMIE can achieve better local control of tumor recurrence compared with MIE. Retrospective studies by Park et al. (22) and Deng et al. (56) reported that RAMIE yielded more dissected lymph nodes than conventional MIE. Chao et al. (20) also showed that compared with MIE, RAMIE resulted in a higher lymph node yield along the left RLN without increasing morbidity. Park et al. demonstrated that RAMIE is associated with better total mediastinal lymphadenectomy, including dissection of the RLN nodes, especially for upper or middle ESCC (79). The rate of locoregional tumor recurrence could be decreased after RAMIE. van der Sluis et al. (80) reported the long-term results of 108 patients who underwent RAMIE. The 5-year OS rate was 42.0%, the median DFS was 21 months, and the median OS was 29 months. Tumor recurrence occurred in 51 patients (49.5%) and was locoregional only in 6 patients (6.0%), systemic only in 31 patients (30.0%), and combined in 14 patients (14.0%). According to their results, RAMIE was oncologically effective and provided excellent local control with a low percentage of local recurrence at long-term follow-up. Weksler et al. (81) reported that there were no differences in the long-term survival of patients with esophageal cancer undergoing RAMIE, MIE, and open esophagectomy. Notably, they considered that the surgeon’s experience and ability might be more critical than the surgical approach for esophageal cancer. Park et al. (22) demonstrated that 5-year OS and recurrence-free survival (RFS) rates were similar between patients undergoing RAMIE and MIE. Yerokun et al. (82) analyzed 4,266 patients in the National Cancer Database who underwent RAMIE (231 cases), MIE (1,077 cases), or open esophagectomy (2,958 cases) for esophageal cancer. Comparison of survival based on surgical procedures showed that: the 3-year OS was not different between RAMIE and MIE for adenocarcinoma, but the 3-year OS for RAMIE was significantly higher than MIE for squamous cell carcinoma (84% vs. 56%, P=0.034). In 2018, Espinoza-Mercado et al. (83) demonstrated that RAMIE and MIE had similar 30- and 90-day mortality rates, as well as long-term survival rates comparable with open esophagectomy, and no significant differences in median OS estimates according to the National Cancer Database.
Summary
In these consensus statements, we can find many details of technique in robot-assisted esophagectomy. We hope they will provide a reference for the beginners and reduce their learning curve. Compared with both traditional open and thoracoscopic surgery, we have yet to see the advantages of RAMIE for oncological outcomes. What are the benefits of RAMIE? We believe that it offers improved surgical efficiency, facilitates a more comfortable surgical experience, and promotes more sophisticated complex surgical techniques, such as precision anastomosis technology. If all of these things are done well, improvements in the quality of treatment received by the entire esophageal cancer population may be achieved.
Acknowledgments
We thank for all experts who have participated in the consensus statement.
Funding: This study is funded by the Gaofeng Clinical Medicine Grant Support of Shanghai Municipal Education Commission. The funding body has no role in the design of the study, data collection and analysis, data interpretation and in writing the manuscript.
Footnote
Reporting Checklist: The authors have completed the AGREE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1945
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1945). ISS reports non-financial support from Intuitive Surgical, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Boone J, Livestro DP, Elias SG, et al. International survey on esophageal cancer: part I surgical techniques. Dis Esophagus 2009;22:195-202. [Crossref] [PubMed]
- Haverkamp L, Seesing MF, Ruurda JP, et al. Worldwide trends in surgical techniques in the treatment of esophageal and gastroesophageal junction cancer. Dis Esophagus 2017;30:1-7. [PubMed]
- Nafteux P, Moons J, Coosemans W, et al. Minimally invasive oesophagectomy: a valuable alternative to open oesophagectomy for the treatment of early oesophageal and gastro-oesophageal junction carcinoma. Eur J Cardiothorac Surg 2011;40:1455-63; discussion 1463-4. [Crossref] [PubMed]
- Sihag S, Wright CD, Wain JC, et al. Comparison of perioperative outcomes following open versus minimally invasive Ivor Lewis oesophagectomy at a single, high-volume centre. Eur J Cardiothorac Surg 2012;42:430-7. [Crossref] [PubMed]
- Mariette C, Markar SR, Dabakuyo-Yonli TS, et al. Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer. N Engl J Med 2019;380:152-62. [Crossref] [PubMed]
- Straatman J, van der Wielen N, Cuesta MA, et al. Minimally Invasive Versus Open Esophageal Resection: Three-year Follow-up of the Previously Reported Randomized Controlled Trial: the TIME Trial. Ann Surg 2017;266:232-36. [Crossref] [PubMed]
- Takeuchi H, Miyata H, Ozawa S, et al. Comparison of Short-Term Outcomes Between Open and Minimally Invasive Esophagectomy for Esophageal Cancer Using a Nationwide Database in Japan. Ann Surg Oncol 2017;24:1821-27. [Crossref] [PubMed]
- Sihag S, Kosinski AS, Gaissert HA, et al. Minimally Invasive Versus Open Esophagectomy for Esophageal Cancer: A Comparison of Early Surgical Outcomes From The Society of Thoracic Surgeons National Database. Ann Thorac Surg 2016;101:1281-8; discussion 1288-9. [Crossref] [PubMed]
- Kernstine KH, DeArmond DT, Karimi M, et al. The robotic, 2-stage, 3-field esophagolymphadenectomy. J Thorac Cardiovasc Surg 2004;127:1847-9. [Crossref] [PubMed]
- van Hillegersberg R, Boone J, Draaisma WA, et al. First experience with robot-assisted thoracoscopic esophagolymphadenectomy for esophageal cancer. Surg Endosc 2006;20:1435-9. [Crossref] [PubMed]
- Ruurda JP, Draaisma WA, van Hillegersberg R, et al. Robot-assisted endoscopic surgery: a four-year single-center experience. Dig Surg 2005;22:313-20. [Crossref] [PubMed]
- Kernstine KH. The first series of completely robotic esophagectomies with three-field lymphadenectomy: initial experience. Surg Endosc 2008;22:2102. [Crossref] [PubMed]
- Anderson C, Hellan M, Kernstine K, et al. Robotic surgery for gastrointestinal malignancies. Int J Med Robot 2007;3:297-300. [Crossref] [PubMed]
- Galvani CA, Gorodner MV, Moser F, et al. Robotically assisted laparoscopic transhiatal esophagectomy. Surg Endosc 2008;22:188-95. [Crossref] [PubMed]
- van der Sluis PC, van der Horst S, May AM, et al. Robot-assisted Minimally Invasive Thoracolaparoscopic Esophagectomy Versus Open Transthoracic Esophagectomy for Resectable Esophageal Cancer: A Randomized Controlled Trial. Ann Surg 2019;269:621-30. [Crossref] [PubMed]
- Chao YK, Li ZG, Wen YW, et al. Robotic-assisted Esophagectomy vs Video-Assisted Thoracoscopic Esophagectomy (REVATE): study protocol for a randomized controlled trial. Trials 2019;20:346. [Crossref] [PubMed]
- Yang Y, Zhang X, Li B, et al. Robot-assisted esophagectomy (RAE) versus conventional minimally invasive esophagectomy (MIE) for resectable esophageal squamous cell carcinoma: protocol for a multicenter prospective randomized controlled trial (RAMIE trial, robot-assisted minimally invasive Esophagectomy). BMC Cancer 2019;19:608. [Crossref] [PubMed]
- Yang Y, Li B, Hua R, et al. Assessment of Quality Outcomes and Learning Curve for Robot-Assisted Minimally Invasive McKeown Esophagectomy. Ann Surg Oncol 2020. Epub ahead of print. [Crossref] [PubMed]
- Akiyama H, Tsurumaru M, Udagawa H, et al. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg 1994;220:364-72; discussion 372-3. [Crossref] [PubMed]
- Chao YK, Hsieh MJ, Liu YH, et al. Lymph Node Evaluation in Robot-Assisted Versus Video-Assisted Thoracoscopic Esophagectomy for Esophageal Squamous Cell Carcinoma: A Propensity-Matched Analysis. World J Surg 2018;42:590-98. [Crossref] [PubMed]
- Deng HY, Luo J, Li SX, et al. Does robot-assisted minimally invasive esophagectomy really have the advantage of lymphadenectomy over video-assisted minimally invasive esophagectomy in treating esophageal squamous cell carcinoma? A propensity score-matched analysis based on short-term outcomes. Dis Esophagus 2019;32:doy110. [Crossref] [PubMed]
- van der Horst S, de Maat MFG, van der Sluis PC, et al. Extended thoracic lymph node dissection in robotic-assisted minimal invasive esophagectomy (RAMIE) for patients with superior mediastinal lymph node metastasis. Ann Cardiothorac Surg 2019;8:218-25. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Cerfolio RJ, Wei B, Hawn MT, et al. Robotic Esophagectomy for Cancer: Early Results and Lessons Learned. Semin Thorac Cardiovasc Surg 2016;28:160-9. [Crossref] [PubMed]
- Orringer MB, Marshall B, Chang AC, et al. Two thousand transhiatal esophagectomies: changing trends, lessons learned. Ann Surg 2007;246:363-72; discussion 372-4. [Crossref] [PubMed]
- Hulscher JB, Tijssen JG, Obertop H, et al. Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta-analysis. Ann Thorac Surg 2001;72:306-13. [Crossref] [PubMed]
- Davies AR, Sandhu H, Pillai A, et al. Surgical resection strategy and the influence of radicality on outcomes in oesophageal cancer. Br J Surg 2014;101:511-7. [Crossref] [PubMed]
- Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002;347:1662-9. [Crossref] [PubMed]
- Dunn DH, Johnson EM, Anderson CA, et al. Operative and survival outcomes in a series of 100 consecutive cases of robot-assisted transhiatal esophagectomies. Dis Esophagus 2017;30:1-7. [Crossref] [PubMed]
- Mori K, Yamagata Y, Aikou S, et al. Short-term outcomes of robotic radical esophagectomy for esophageal cancer by a nontransthoracic approach compared with conventional transthoracic surgery. Dis Esophagus 2016;29:429-34. [Crossref] [PubMed]
- Nakauchi M, Uyama I, Suda K, et al. Robot-assisted mediastinoscopic esophagectomy for esophageal cancer: the first clinical series. Esophagus 2019;16:85-92. [Crossref] [PubMed]
- Daiko H, Nishimura M. A pilot study of the technical and oncologic feasibility of thoracoscopic esophagectomy with extended lymph node dissection in the prone position for clinical stage I thoracic esophageal carcinoma. Surg Endosc 2012;26:673-80. [Crossref] [PubMed]
- Hodari A, Park KU, Lace B, et al. Robot-Assisted Minimally Invasive Ivor Lewis Esophagectomy With Real-Time Perfusion Assessment. Ann Thorac Surg 2015;100:947-52. [Crossref] [PubMed]
- Sarkaria IS, Rizk NP. Robotic-assisted minimally invasive esophagectomy: the Ivor Lewis approach. Thorac Surg Clin 2014;24:211-22. vii. [Crossref] [PubMed]
- de la Fuente SG, Weber J, Hoffe SE, et al. Initial experience from a large referral center with robotic-assisted Ivor Lewis esophagogastrectomy for oncologic purposes. Surg Endosc 2013;27:3339-47. [Crossref] [PubMed]
- Salem AI, Thau MR, Strom TJ, et al. Effect of body mass index on operative outcome after robotic-assisted Ivor-Lewis esophagectomy: retrospective analysis of 129 cases at a single high-volume tertiary care center. Dis Esophagus 2017;30:1-7. [PubMed]
- Abbott A, Shridhar R, Hoffe S, et al. Robotic assisted Ivor Lewis esophagectomy in the elderly patient. J Gastrointest Oncol 2015;6:31-8. [PubMed]
- Trugeda S, Fernandez-Diaz MJ, Rodriguez-Sanjuan JC, et al. Initial results of robot-assisted Ivor-Lewis oesophagectomy with intrathoracic hand-sewn anastomosis in the prone position. Int J Med Robot 2014;10:397-403. [Crossref] [PubMed]
- Markar SR, Wiggins T, Antonowicz S, et al. Minimally invasive esophagectomy: Lateral decubitus vs. prone positioning; systematic review and pooled analysis. Surg Oncol 2015;24:212-9. [Crossref] [PubMed]
- Zhang Y, Han Y, Gan Q, et al. Early Outcomes of Robot-Assisted Versus Thoracoscopic-Assisted Ivor Lewis Esophagectomy for Esophageal Cancer: A Propensity Score-Matched Study. Ann Surg Oncol 2019;26:1284-91. [Crossref] [PubMed]
- Zhang X, Su Y, Yang Y, et al. Robot assisted esophagectomy for esophageal squamous cell carcinoma. J Thorac Dis 2018;10:3767-75. [Crossref] [PubMed]
- Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg 1982;69:613-6. [Crossref] [PubMed]
- Akiyama Y, Iwaya T, Endo F, et al. Thoracoscopic esophagectomy with total meso-esophageal excision reduces regional lymph node recurrence. Langenbecks Arch Surg 2018;403:967-75. [Crossref] [PubMed]
- Schurink B, Defize IL, Mazza E, et al. Two-Field Lymphadenectomy During Esophagectomy: The Presence of Thoracic Duct Lymph Nodes. Ann Thorac Surg 2018;106:435-39. [Crossref] [PubMed]
- Yoshida N, Nagai Y, Baba Y, et al. Effect of Resection of the Thoracic Duct and Surrounding Lymph Nodes on Short- and Long-Term and Nutritional Outcomes After Esophagectomy for Esophageal Cancer. Ann Surg Oncol 2019;26:1893-900. [Crossref] [PubMed]
- Cuesta MA, Weijs TJ, Bleys RL, et al. A new concept of the anatomy of the thoracic oesophagus: the meso-oesophagus. Observational study during thoracoscopic esophagectomy. Surg Endosc 2015;29:2576-82. [Crossref] [PubMed]
- Anand S, Kalayarasan R, Chandrasekar S, et al. Minimally Invasive Esophagectomy with Thoracic Duct Resection Post Neoadjuvant Chemoradiotherapy for Carcinoma Esophagus-Impact on Lymph Node Yield and Hemodynamic Parameters. J Gastrointest Cancer 2019;50:230-35. [Crossref] [PubMed]
- Hou X, Fu JH, Wang X, et al. Prophylactic thoracic duct ligation has unfavorable impact on overall survival in patients with resectable oesophageal cancer. Eur J Surg Oncol 2014;40:1756-62. [Crossref] [PubMed]
- Chen JY, Liu QW, Zhang SS, et al. Prophylactic thoracic duct ligation is associated with poor prognosis and regional lymph node relapse in esophageal squamous cell carcinoma. J Surg Oncol 2020;122:336-43. [Crossref] [PubMed]
- Boone J, Schipper ME, Moojen WA, et al. Robot-assisted thoracoscopic oesophagectomy for cancer. Br J Surg 2009;96:878-86. [Crossref] [PubMed]
- Wee JO, Bravo-Iniguez CE, Jaklitsch MT. Early Experience of Robot-Assisted Esophagectomy With Circular End-to-End Stapled Anastomosis. Ann Thorac Surg 2016;102:253-9. [Crossref] [PubMed]
- Honda M, Kuriyama A, Noma H, et al. Hand-sewn versus mechanical esophagogastric anastomosis after esophagectomy: a systematic review and meta-analysis. Ann Surg 2013;257:238-48. [Crossref] [PubMed]
- Kim RH, Takabe K. Methods of esophagogastric anastomoses following esophagectomy for cancer: A systematic review. J Surg Oncol 2010;101:527-33. [Crossref] [PubMed]
- Liu QX, Min JX, Deng XF, et al. Is hand sewing comparable with stapling for anastomotic leakage after esophagectomy? A meta-analysis. World J Gastroenterol 2014;20:17218-26. [Crossref] [PubMed]
- Kamarajah SK, Bundred JR, Singh P, et al. Anastomotic techniques for oesophagectomy for malignancy: systematic review and network meta-analysis. BJS Open 2020;4:563-76. [Crossref] [PubMed]
- Deng HY, Huang WX, Li G, et al. Comparison of short-term outcomes between robot-assisted minimally invasive esophagectomy and video-assisted minimally invasive esophagectomy in treating middle thoracic esophageal cancer. Dis Esophagus 2018. [Crossref] [PubMed]
- Weksler B, Sharma P, Moudgill N, et al. Robot-assisted minimally invasive esophagectomy is equivalent to thoracoscopic minimally invasive esophagectomy. Dis Esophagus 2012;25:403-9. [Crossref] [PubMed]
- Yang Y, Zhang X, Li B, et al. Short- and mid-term outcomes of robotic versus thoraco-laparoscopic McKeown esophagectomy for squamous cell esophageal cancer: a propensity score-matched study. Dis Esophagus 2020;33:doz080. [Crossref] [PubMed]
- van Rijswijk AS, Hagens ERC, van der Peet DL, et al. Differences in Esophageal Cancer Surgery in Terms of Surgical Approach and Extent of Lymphadenectomy: Findings of an International Survey. Ann Surg Oncol 2019;26:2063-72. [Crossref] [PubMed]
- Nafteux P, Depypere L, Van Veer H, et al. Principles of esophageal cancer surgery, including surgical approaches and optimal node dissection (2- vs. 3-field). Ann Cardiothorac Surg 2017;6:152-58. [Crossref] [PubMed]
- Tachimori Y, Ozawa S, Numasaki H, et al. Efficacy of lymph node dissection by node zones according to tumor location for esophageal squamous cell carcinoma. Esophagus 2016;13:1-7. [Crossref] [PubMed]
- Udagawa H, Ueno M, Shinohara H, et al. The importance of grouping of lymph node stations and rationale of three-field lymphoadenectomy for thoracic esophageal cancer. J Surg Oncol 2012;106:742-7. [Crossref] [PubMed]
- Kim DJ, Park SY, Lee S, et al. Feasibility of a robot-assisted thoracoscopic lymphadenectomy along the recurrent laryngeal nerves in radical esophagectomy for esophageal squamous carcinoma. Surg Endosc 2014;28:1866-73. [Crossref] [PubMed]
- Chen J, Cai W, Lin Y, et al. Patterns and rates of abdominal lymphatic metastasis following esophageal carcinoma. PLoS One 2017;12:e0185424. [Crossref] [PubMed]
- Ozawa S, Koyanagi K, Ninomiya Y, et al. Postoperative complications of minimally invasive esophagectomy for esophageal cancer. Ann Gastroenterol Surg 2020;4:126-34. [Crossref] [PubMed]
- Shanmugasundaram R, Hopkins R, Neeman T, et al. Minimally invasive McKeown’s vs open oesophagectomy for cancer: A meta-analysis. Eur J Surg Oncol 2019;45:941-9. [Crossref] [PubMed]
- Sarkaria IS, Rizk NP, Goldman DA, et al. Early Quality of Life Outcomes After Robotic-Assisted Minimally Invasive and Open Esophagectomy. Ann Thorac Surg 2019;108:920-8. [Crossref] [PubMed]
- Williams VA, Watson TJ, Zhovtis S, et al. Endoscopic and symptomatic assessment of anastomotic strictures following esophagectomy and cervical esophagogastrostomy. Surg Endosc 2008;22:1470-6. [Crossref] [PubMed]
- Wong J, Cheung H, Lui R, et al. Esophagogastric anastomosis performed with a stapler: the occurrence of leakage and stricture. Surgery 1987;101:408-15. [PubMed]
- Wang WP, Gao Q, Wang KN, et al. A prospective randomized controlled trial of semi-mechanical versus hand-sewn or circular stapled esophagogastrostomy for prevention of anastomotic stricture. World J Surg 2013;37:1043-50. [Crossref] [PubMed]
- Rice TW. Anastomotic stricture complicating esophagectomy. Thorac Surg Clin 2006;16:63-73. [Crossref] [PubMed]
- van Heijl M, Gooszen JA, Fockens P, et al. Risk factors for development of benign cervical strictures after esophagectomy. Ann Surg 2010;251:1064-9. [Crossref] [PubMed]
- Park S, Hyun K, Lee HJ, et al. A study of the learning curve for robotic oesophagectomy for oesophageal cancer. Eur J Cardiothorac Surg 2018;53:862-70. [Crossref] [PubMed]
- Hernandez JM, Dimou F, Weber J, et al. Defining the learning curve for robotic-assisted esophagogastrectomy. J Gastrointest Surg 2013;17:1346-51. [Crossref] [PubMed]
- Sarkaria IS, Rizk NP, Grosser R, et al. Attaining Proficiency in Robotic-Assisted Minimally Invasive Esophagectomy While Maximizing Safety During Procedure Development. Innovations (Phila) 2016;11:268-73. [Crossref] [PubMed]
- Park SY, Kim DJ, Kang DR, et al. Learning curve for robotic esophagectomy and dissection of bilateral recurrent laryngeal nerve nodes for esophageal cancer. Dis Esophagus 2017;30:1-9. [Crossref] [PubMed]
- van der Sluis PC, Ruurda JP, van der Horst S, et al. Learning Curve for Robot-Assisted Minimally Invasive Thoracoscopic Esophagectomy: Results From 312 Cases. Ann Thorac Surg 2018;106:264-71. [Crossref] [PubMed]
- Zhang H, Chen L, Wang Z, et al. The Learning Curve for Robotic McKeown Esophagectomy in Patients With Esophageal Cancer. Ann Thorac Surg 2018;105:1024-30. [Crossref] [PubMed]
- Park SY, Kim DJ, Do YW, et al. The Oncologic Outcome of Esophageal Squamous Cell Carcinoma Patients After Robot-Assisted Thoracoscopic Esophagectomy With Total Mediastinal Lymphadenectomy. Ann Thorac Surg 2017;103:1151-7. [Crossref] [PubMed]
- van der Sluis PC, Ruurda JP, Verhage RJ, et al. Oncologic Long-Term Results of Robot-Assisted Minimally Invasive Thoraco-Laparoscopic Esophagectomy with Two-Field Lymphadenectomy for Esophageal Cancer. Ann Surg Oncol 2015;22 Suppl 3:S1350-6. [Crossref] [PubMed]
- Weksler B, Sullivan JL. Survival After Esophagectomy: A Propensity-Matched Study of Different Surgical Approaches. Ann Thorac Surg 2017;104:1138-46. [Crossref] [PubMed]
- Yerokun BA, Sun Z, Yang CJ, et al. Minimally Invasive Versus Open Esophagectomy for Esophageal Cancer: A Population-Based Analysis. Ann Thorac Surg 2016;102:416-23. [Crossref] [PubMed]
- Espinoza-Mercado F, Imai TA, Borgella JD, et al. Does the Approach Matter? Comparing Survival in Robotic, Minimally Invasive, and Open Esophagectomies. Ann Thorac Surg 2019;107:378-85. [Crossref] [PubMed]