The prognostic value of peak arterial lactate levels within 72 h of lung transplantation in identifying patient outcome
Introduction
Lactic acidosis is associated with tissue hypoperfusion, which is often induced by hypoxia, and is a predictor of poor outcome in surgical patients admitted to the intensive care unit (ICU). Under anaerobic conditions, cellular metabolism converts pyruvate to lactate. The lactic acid levels objectively correspond to the severity of tissue hypoperfusion, and lactic acidosis or hyperlactatemia may be a unique marker of circulatory failure.
As a common biomarker in critically ill patients, hyperlactatemia is considered to be a prognostic factor in sepsis, trauma, shock, and post-cardiac surgery (1-5). Several reports suggest increased lactate may contribute to the predictive value of postoperative lactate levels in regard to outcome. A growing number of previous studies have focused on investigating the relationship between lactate and postoperative morbidity and mortality and have summarized possible underlying mechanisms (6). Moreover, hyperlactatemia was associated with longer times to extubation, longer lengths of stay, and acute kidney injury after lung transplantation (LTx) (7). Some researchers have proposed that, in addition to hypoperfusion, the degree of hemodilution and lower peripheral oxygen delivery results in tissue hypoxia and is likely to cause a reduction in lactate clearance. However, few studies have focused on the effect of elevated lactate levels on outcome after LTx.
Hyperlactatemia in critical illness is considered to be a feature of inadequate oxygen delivery and consequent anaerobic metabolism (8). Oxidative phosphorylation is impaired in hypoxic conditions resulting in accumulations of pyruvate and, following catalytic reduction, lactate (9). Other causes of elevated lactate production and reduced lactate clearance include inadequate oxygen delivery and the stress response, which are mediated through the acute phase immune response and result in endocrine and metabolic changes. Persistent hyperlactatemia is always found to be linked to microcirculatory derangement and contributes to higher morbidity and mortality (10). To our best knowledge, several mechanisms can lead to an altered production/clearance balance and lactate accumulation during and after LTx. It is also common to find peripheral hypoperfusion [due to low cardiac output, high central venous pressure (CVP) or vasoconstriction], hypoxemia, anemia, activation of the sympathetic drive, and organ dysfunction, some of which may impair oxygen delivery causing microcirculatory disturbance, which aggravates disease progression and leads to a worse prognosis.
Despite abundant investigations showing that lactate may play a significant role in predicting the risk factors for poor outcome in various conditions, there has been no exploration of the relationship between postoperative peak arterial lactate levels (PL) and 30-day mortality and late mortality in LTx patients. The primary aim of the present research was to explore the impact of demographic and clinical factors on all-cause mortality for LTx patients at our facility. In particular, we wanted to assess whether postoperative PL within 72 h of LTx can be used as a prognostic biomarker of short and longer-term mortality in our single-center cohort.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-3445).
Methods
Study design
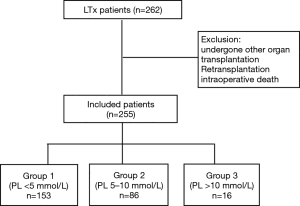
In this retrospective study, we analyzed all patients who underwent LTx at the Affiliated Wuxi People’s Hospital of Nanjing Medical University, between January 2015 and September 2017. Inclusion criteria for this analysis were all adult patients (aged >18 years) who were evaluated for LTx. Exclusion criteria included any patient who had undergone other organ transplantation, retransplantation, or those who had suffered intraoperative death. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Wuxi People’s Hospital Affiliated to Nanjing Medical University (KYLLH2018024) and informed consent was taken from all the patients. The institutional database contains prospectively collected data, including all clinical characteristics, risk factors, and laboratory data of all patients who received LTx at the facility.
We defined PL within 72 h as the target factor in the present analysis. Following surgery, patients were immediately transported to ICU where serial measurements of blood lactate are monitored as standard care for all patients. In the following conditions we measured lactate levels repeatedly: the degree of hyperlactatemia and hemodynamic instability. According to our measurements, we divided patients into three groups: Group 1 (PL <5 mmol/L); Group 2 (PL =5–10 mmol/L); Group 3 (PL >10 mmol/L).
The primary endpoint of the study was 30-day mortality, defined as all-cause mortality within 30 days postoperatively, and late mortality was defined as all-cause mortality beyond 30 days. Follow-up data of late mortality was collected from the database of the LTx center in the authors’ institution (http://clutr.cotr.cn).
Anesthetic procedure
Every patient was subjected to the LTx anesthesia protocol at Wuxi People’s Hospital, which comprises standard perioperative medical treatment and a routine ventilation protocol. Anesthesia induction protocol was intravenous injection with midazolam, sufentanil, etomidate, and cisatracurium besilat and maintained with administration of propofol, cisatracurium besilat, remifentanil, and sevoflurane. All patients received two deep venipuncture, a radial artery catheter and peripherally inserted central catheter, then a Swan-Ganz catheter was inserted into the right internal jugular vein to continuously monitor CVP, mean pulmonary artery pressure (MPAP), cardiac index (CI), systemic vascular resistance (SVR), and pulmonary vascular resistance (PVR). The decision whether to use extracorporeal membrane oxygenation (ECMO) relied on the assessment of blood gas analysis and the degree of pulmonary artery pressure. The depth of anesthesia was monitored with a bispectral index monitor (Philips Medical System, America). Ventilation protocol was performed to set tidal volumes between 6 and 10 mL/kg. If necessary, vasoconstrictor drugs such as norepinephrine, phenylephrine, or epinephrine were used to maintain hemodynamic stability.
ICU management
In our institution, postoperative care of patients is standardized and managed in the ICU. In this setting, blood samples were obtained at the prescribed time, including blood gas analysis. We use epinephrine (0.1–1 µg/kg·min), milrinone (0.25–1 µg/kg·min), noradrenaline (0.01–1 µg/kg·min), or dopamine (2–10 µg/kg·min) to support hemodynamic stability, in order to achieve a mean arterial pressure goal of 65 to 70 mmHg.
Statistical analysis
Data are presented as mean ± standard deviation, or as a percentage. Binary logistic regression was used to assess the levels of PL in predicting 30-day mortality. Cox proportional hazard regression was used to evaluate the utility of PL in determining late mortality. If variables with a P value <0.05, risk factors were entered to the multivariate logistic and Cox regression analyses. The Kaplan-Meier method was performed to assess cumulative survival. We reported odds ratios and hazards by 95% confidence intervals. All tests were two-sided and a P value <0.05 was considered significant. As several missing values for the lactate levels after 72 h in the study. The missing values were substituted by interpolating from two adjacent data points and extrapolating from two previously observed data points. Analyses were performed using the SPSS statistics 22 package (IBM, Chicago, USA).
Results
Patient characteristics
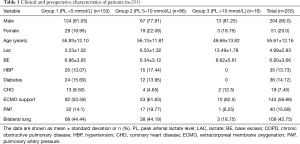
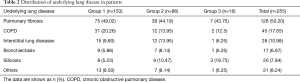
During the study period, 262 patients had LTx and were admitted to the ICU at the Affiliated Wuxi People’s Hospital of Nanjing Medical University. The maximal follow-up was 1,121 days. Four patients received postoperative retransplantation, while three patients underwent LTx 2 years later. A final 255 patients who met the inclusion criteria were included in the study (Figure 1). Table 1 shows the clinical and preoperative characteristics of the three groups. The overall mean PL and BE (base excess) values were 4.99±2.93 and 6.80±3.66, respectively. The average age was 55.61±12.16 years and 80% (n=204) of patients were male. Comorbidities were also detected: 13.73% (n=35) of patients had known hypertension, 7.45% (n=19) had coronary heart disease, and 14.12% (n=36) suffered diabetes. Overall, 56.86% of patients needed ECMO to maintain an oxygen supply. Forty patients (15.69%) of the study population had a complication of pulmonary hypertension (PH), and 42.75% of these received bilateral LTx. Table 2 shows the distribution of underlying lung disease in all patients.
Full table
Full table
Lactic acidosis and early mortality
The relationship between PL and 30-day mortality is shown in Table 3. Compared with Group 1, 30-day mortality was significantly increased in the intermediate- and high-lactate groups. The 30-day mortality rate was 17.9% in Group 1 (PL <5 mmol/L), 28.9% in Group 2 (PL =5–10 mmol/L), and 68.8% in Group 3 (PL >10 mmol/L).

Full table
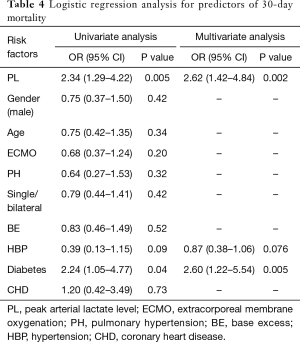
Risk factors identified with 30-day mortality using logistic regression analysis are shown in Table 4. In addition to PL, other variables such as gender, age, ECMO support, pulmonary artery pressure (PAP), single or bilateral LTx, peak BE value, hypertension, diabetes, and coronary heart disease were included in the univariate analyses to predict 30-day mortality. If the P value was <0.05, multivariate logistic regression analyses were performed to assess these factors. Subsequently, PL, age, and diabetes were identified as independent risk factors for 30-day mortality.
Full table
Univariate Cox regression identified PL as a risk factor for late mortality (Table 5). Likewise, diabetes was considered to be a risk factor. Subsequent multivariate analysis confirmed both PL and diabetes as independent risk factors for late mortality.
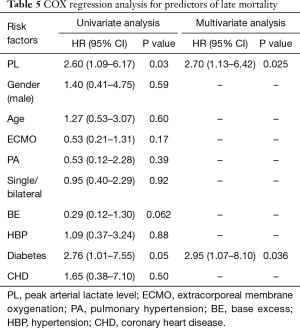
Full table
The lactate value distribution and outcome
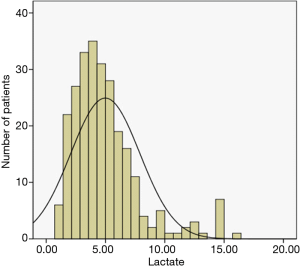
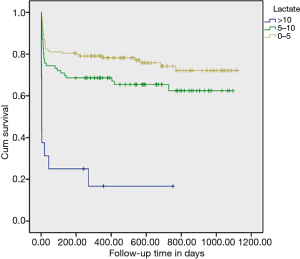
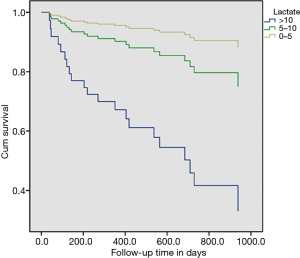
The distribution of lactate value is presented in Figure 2, and of the admitted patients, 102 (40%) had hyperlactatemia (PL >5 mmol/L) after LTx. In Figure 3, 33.73% of those with PL <5–10 mmol/L and 6.27% with PL >5 mmol/L, the Kaplan-Meier curves shows postoperative survival stratified by PL in LTx patients. While Figure 4 shows the corrected survival curves for late survival in the three groups (P<0.05).
Discussion
The present study investigated the role of PL within 72 h of LTx in predicting 30-day and late mortality. We found increased arterial lactate was associated with a significant risk of death. In logistic regression, the PL level as well as diabetes was associated with 30-day mortality. In multivariate regression analyses, PL was an independent predictor of poor outcome, which was related to late mortality. The incidence of 30-day mortality in the three groups was 16.99%, 25.58% and 68.8% respectively, which suggested elevated lactate had a prognostic value for short-term mortality.
Prior research has documented that the perioperative lactate level in high-risk patients shows that postoperative hyperlactatemia is associated with a disorder of intraoperative oxygen delivery and consumption, an “oxygen debt” (11) and/or a reduction in tissue ability to extract oxygen (12). Recent studies indicate that an impaired microcirculation function with an increased lactate level contributes to poor outcome in postoperative patients and critically ill patients (4,6,7,12). In our findings, PL as a unique predictive risk factor was significantly related to 30-day and late mortality in LTx patients, which is in line with previous findings. Felten et al. (13) have provided evidence of the association between hyperlactatemia and postoperative allograft dysfunction after bilateral LTx in cystic fibrosis patients, reporting that hyperlactatemia reflects the severity of early primary graft dysfunction which is responsible for significant morbidity and mortality after LTx. To some extent our results were also in agreement with their opinions. Research involving patients who received liver transplantation suggested initial postoperative lactate values were moderately predictive of 30-day and in-hospital mortality (14-16). In addition, postoperative peak hyperlactatemia at 24 hours was associated with higher in-hospital and long-term mortality in patients after cardiac surgery when hyperlactatemia was defined as a lactate value over 3.0 mmol/L (17). Furthermore, increased arterial blood lactate levels during the first 24 hours in the ICU following gastrointestinal surgery showed a strong association with an increased risk of death (18). It is mentioned in the literature early complications and mortality after LTx, while previous studies indicated that perioperative factors and management also affected the long-term prognosis (19). In the study, we chose the postoperative PL within 72 h postoperatively based on our work experience. Besides, we also consider additional hemodynamic events occurring in the ICU postoperatively
However, there have been few reports focusing on the relationship between lactate and mortality in LTx patients, and this has prompted the current study. A retrospective study in one of the largest Japanese single-center cohorts revealed that hyperlactatemia occurred after heart transplantation in 59.2% of the population but was not associated with unfavorable outcomes other than pulmonary complications (20). They also found respiratory dysfunction after transplantation due to deconditioning of the respiratory muscles before transplantation. Moreover, Meyer et al. found that patients with heart failure had a decreased respiratory muscle function, which causes a poorer outcome (21). We hypothesize that LTx patients with cardiorespiratory dysfunction who have poor exercise tolerance are prone to develop postoperative hyperlactatemia and may be likely to have a worse prognosis. Patients undergoing LTx could be considered as a relatively different category of patient, due to the nature of the postoperative pathophysiological process, such as ischemia reperfusion injury and graft rejection. It is conceivable that anaerobic metabolism from a local oxygen deficit and a state of accelerated glycolysis might result in a high lactate concentration.
We showed a 30-day mortality rate of 23.14% during the study period in our center, whereas Thabut (22) reported a 3-month mortality rate of only 11%. A larger drop in early survival in the 30 days following LTx might be due to the graft quality. Improvements in the management of LTx patients in the early postoperative period are expected to narrow the gap in future. Single and bilateral LTx has evolved as two therapeutic options in patients with end stage chronic lung disease refractory to medical management. The Statistics of the International Society for Heart and Lung Transplantation (ISHLT) registry has suggested that survival is better in bilateral compared with single-lung transplant recipients (23). Our study revealed no difference in early and late mortality between double and single LTx, which is in accordance with findings reported by the American Thoracic Society and the European Respiratory Society (24). Furthermore, whether LTx with intraoperative ECMO support is beneficial for patients with PH or is related to poor outcome remains controversial (25-28). Our data have not shown any definitive evidence of the detrimental nature of ECMO support in the presence of pulmonary hypertension, although these associations are confounded by the differences between limited populations, particularly in regard to the patients’ underlying conditions.
As hypoperfusion, inflammatory reaction and ischemia-reperfusion injury occurred during the perioperative period of LTx, it seems reasonable that hyperlactatemia developed. Early arterial lactate measurement was useful in guiding the hemodynamic optimization, while sustained hyperlactatemia may contribute to organ failure, which is harder to treat (10). Several other etiological factors may cause an elevated lactate level leading to hyperlactatemia including systemic inflammatory reaction, microcirculation, tissue hypoxia, mitogenic factors as well as unrecognized concomitant infections, septic shock, and some forms of mesenteric ischemia (29). Patients with or without hyperlactatemia showed similar demographic, clinical, and laboratory variables even though their prognosis was significantly different, suggesting that hyperlactatemia is a prominent marker independent of any other clinical variables. Serum lactate reflects a balance of its production and clearance, which in the case of LTx could be impaired, contributing to lactic acid accumulation.
It is worth discussing the interesting finding that patients with diabetes suffered a greater risk of 30-day and long-term mortality, which is also consistent with previous research (30). Glucose and lactate are interrelated components of the carbohydrate metabolism, each with the capacity to serve as a precursor for the biosynthesis of the other (31). Higher lactate levels correspond with higher glucose levels. Lactatemia co-occurring with hyperglycemia in cardiac surgery (32) is considered to be a better predictor of mortality than hyperglycemia alone (33,34). Extreme metabolic responses to surgical stress, such as hyperglycemia and hyperlactatemia, have been associated with worse outcomes after major operations and in critical illness. Hypoxia and ischemia cause inhibition of mitochondrial oxidative phosphorylation, which prevents the complete oxidation of glucose. Consequently, anaerobic glycolysis becomes the primary source of energy for the cells. Enhanced anaerobic glycolysis causes the accumulation of lactate. Lactate is therefore considered to be a useful biomarker of tissue hypoxia.
Our study suffers from some limitations. First, this retrospective study had a relatively small number of patients, and the follow-up period was limited which did not allow us to accurately differentiate outcome over a longer period. The long-term outcome for patients needs further study. Second, because of the observational retrospective nature of this study which was based on a single hospital experience, the results may lack universality. Also, there may have been some unknown confounders during the ICU stay that affected the outcome for some patients. Therefore, our results must be interpreted with a degree of caution. Third, lactate clearance was not investigated in our study, since a small number of patients lacked arterial blood samples for the initial lactate measurement. Our finding that patients with diabetes had a greater risk of mortality warrants further research to elucidate the role of postoperative hyperglycemia in LTx patients and the origin and functional significance of glycemic control, which may direct future therapy and outcome. As such, the data presented here could be promising for therapeutic applications.
Conclusions
In conclusion, our findings revealed that the level of postoperative peak lactate within 72 h of LTx was an independent risk factor in predicting 30-day and later mortality, regardless of the initial lactate level and the presence of lactate clearance. In the future, long-term multi-center investigations with larger samples and a greater number of predictive variables should be conducted in order to enhance prognosis and improve the clinical outcome for patients.
Acknowledgments
The authors thank Professor Jingyu Chen and the National Registration Centre of Lung Transplantation Data. We are grateful to all investigators of the study for the data collection. And we also thank American Journal Experts (www.aje.cn) for its linguistic assistance during the preparation of this manuscript.
Funding: The study was supported by funds from the Medical Science Innovation Team Foundation of Wuxi (CXTD001), and the Program of Wuxi Science and Technology Development Plan (CSE31N1615).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-3445
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-3445
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-3445). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Wuxi People’s Hospital Affiliated to Nanjing Medical University (KYLLH2018024) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pruinelli L, Westra BL, Yadav P, et al. Delay Within the 3-Hour Surviving Sepsis Campaign Guideline on Mortality for Patients With Severe Sepsis and Septic Shock. Crit Care Med 2018;46:500-5. [Crossref] [PubMed]
- Fernando SM, Barnaby DP, Herry CL, et al. Helpful Only When Elevated Initial Serum Lactate in Stable Emergency Department Patients with Sepsis Is Specific but Not Sensitive for Future Deterioration. J Emerg Med 2018;54:766-73. [Crossref] [PubMed]
- Raux M, Le Manach Y, Gauss T, et al. Comparison of the Prognostic Significance of Initial Blood Lactate and Base Deficit in Trauma Patients. Anesthesiology 2017;126:522-33. [Crossref] [PubMed]
- Ospina-Tascón GA, Madriñán HJ. Combination of O2 and CO2-derived variables to detect tissue hypoxia in the critically ill patient. J Thorac Dis 2019;11:S1544-50. [Crossref] [PubMed]
- Lopez-Delgado JC, Esteve F, Javierre C, et al. Evaluation of Serial Arterial Lactate Levelsasa Predictor of Hospital and Long-Term Mortality in Patients After Cardiac Surgery. Vet Anaesth Analg 2014;41:498-505.
- Andersen LW, Holmberg MJ, Doherty M, et al. Postoperative Lactate Levels and Hospital Length of Stay After Cardiac Surgery. J Cardiothorac Vasc Anesth 2015;29:1454-60. [Crossref] [PubMed]
- Worrell SG, Haug K, Dubovoy A, et al. Is Lactic Acidosis After Lung Transplantation Associated With Worse Outcomes? Ann Thorac Surg 2020;110:434-40. [Crossref] [PubMed]
- Kraut JA, Madias NE. Lactic acidosis. N Engl J Med 2014;371:2309-19. [Crossref] [PubMed]
- Esteban-Martínez L, Sierra-Filardi E, McGreal RS, et al. Programmed mitophagy is essential for the glycolytic switch during cell differentiation. EMBO J 2017;36:1688-706. [Crossref] [PubMed]
- Kiyatkin ME, Bakker J. Lactate and microcirculation as suitable targets for hemodynamic optimization in resuscitation of circulatory shock. Curr Opin Crit Care 2017;23:348-54. [Crossref] [PubMed]
- Waxman K, Nolan LS, Shoemaker WC. Sequential perioperative lactate determination. Physiological and clinical implications. Crit Care Med 1982;10:96-9. [Crossref] [PubMed]
- Lugo G, Arizpe D, Domínguez G, et al. Relationship between oxygen consumption and oxygen delivery during anesthesia in high-risk surgical patients. Crit Care Med 1993;21:64-9. [Crossref] [PubMed]
- Felten ML, Sinaceur M, Treilhaud M, et al. Factors associated with early graft dysfunction in cystic fibrosis patients receiving primary bilateral lung transplantation. Eur J Cardiothorac Surg 2012;41:686-90. [Crossref] [PubMed]
- Cardoso NM, Silva T, Basile-Filho A, et al. A new formula as a predictive score of post-liver transplantation outcome postoperative MELD-lactate. Transplant Proc 2014;46:1407-12. [Crossref] [PubMed]
- Kim S, Zerillo J, Tabrizian P, et al. Postoperative MELD-Lactate and isolated lactate values as outcome predictors following orthotopic liver transplantation. Shock 2017;48:36-42. [Crossref] [PubMed]
- Patrono D, Romagnoli R. Postreperfusion syndrome, hyperkalemia and machine perfusion in liver transplantation. Transl Gastroenterol Hepatol 2019;4:68. [Crossref] [PubMed]
- Haanschoten MC, Kreeftenberg HG, Arthur Bouwman R, et al. Use of Postoperative Peak Arterial Lactate Level to Predict Outcome After Cardiac Surgery. J Cardiothorac Vasc Anesth 2017;31:45-53. [Crossref] [PubMed]
- Creagh-Brown BC, De Silva AP, Ferrando-Vivas P, et al. Relationship Between Peak Lactate and Patient Outcome Following High-Risk Gastrointestinal Surgery: Influence of the Nature of Their Surgery: Elective Versus Emergency. Crit Care Med 2016;44:918-25. [Crossref] [PubMed]
- Kao CC, Parulekar AD. Postoperative management of lung transplant recipients. J Thorac Dis 2019;11:S1782-S1788. [Crossref] [PubMed]
- Hoshino Y, Kinoshita O, Ono M. The Incidence, Risk Factors, and Outcomes of Hyperlactatemia after Heart Transplantation, One Center’s Experience. Int Heart J 2018;59:81-6. [Crossref] [PubMed]
- Meyer FJ, Borst MM, Zugck C, et al. Respiratory muscle dysfunction in congestive heart failure: clinical correlation and prognostic significance. Circulation 2001;103:2153-8. [Crossref] [PubMed]
- Thabut G, Mal H. Outcomes after Lung Transplantation. J Thorac Dis 2017;9:2684-91. [Crossref] [PubMed]
- Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th adult lung and heart-lung transplant report-2012. J Heart Lung Transplant 2012;31:1073-86. [Crossref] [PubMed]
- Celli BR, Decramer M, Wedzicha JA, et al. An official American Thoracic Society/European Respiratory Society statement: research questions in COPD. Eur Respir J 2015;45:879-905. [Crossref] [PubMed]
- Hoetzenecker K, Schwarz S, Muckenhuber M, et al. Intraoperative ECMO and the possibility of postoperative prolongation improve survival in bilateral lung transplantation. J Thorac Cardiovasc Surg 2018;155:2193-2206.e3. [Crossref] [PubMed]
- Cosgun T, Tomaszek S, Opitz I, et al. Single-center experience with intraoperative extracorporeal membrane oxygenation use in lung transplantation. Int J Artif Organs 2017. Epub ahead of print. [Crossref] [PubMed]
- Julliard WA, Meyer KC, De Oliveira NC, et al. The presence or severity of pulmonary hypertension does not affect outcomes for single-lung transplantation. Thorax 2016;71:478-80. [Crossref] [PubMed]
- Andersen KH, Schultz HH, Nyholm B, et al. Pulmonary hypertension as a risk factor of mortality after lung transplantation. Clin Transplant 2016;30:357-64. [Crossref] [PubMed]
- Isfordink CJ, Dekker D, Monkelbaan JF. Clinical value of serum lactate measurement in diagnosing acute mesenteric ischaemia. Neth J Med 2018;76:60-4. [PubMed]
- Greco G, Kirkwood KA, Gelijns AC, et al. Diabetes Is Associated With Reduced Stress Hyperlactatemia in Cardiac Surgery. Diabetes Care 2018;41:469-77. [Crossref] [PubMed]
- Adeva-Andany M, López-Ojén M, Funcasta-Calderón R, et al. Comprehensive review on lactate metabolismin human health. Mitochondrion 2014;17:76-100. [Crossref] [PubMed]
- Lim JY, Kim JB, Jung SH, et al. Risk factor analysis for nonocclusive mesenteric ischemia following cardiac surgery: A case-control study. Medicine (Baltimore) 2017;96:e8029. [Crossref] [PubMed]
- Kaukonen KM, Bailey M, Egi M, et al. Stress hyperlactatemia modifies the relationship between stress hyperglycemia and outcome: a retrospective observational study. Crit Care Med 2014;42:1379-85. [Crossref] [PubMed]
- Green JP, Berger T, Garg N, et al. Hyperlactatemia affects the association of hyperglycemia with mortality in nondiabetic adults with sepsis. Acad Emerg Med 2012;19:1268-75. [Crossref] [PubMed]
(English Language Editor: D. Fitzgerald)