
Effects of enteral nutrition support combined with enhanced recovery after surgery on the nutritional status, immune function, and prognosis of patients with esophageal cancer after Ivor-Lewis operation
Introduction
Esophageal cancer (EC) is a highly malignant digestive tract tumor, increasing by 450,000 new cases per year worldwide (1). EC comprises esophageal squamous cell carcinoma (ESCC) and adenocarcinoma. China has a high incidence of EC, of which more than 95% are ESCC (2). Drinking, smoking and inflammation leading to chronic irritation are the major risk factors for EC (3). The generally accepted treatments for EC include surgical resection, chemotherapy and radiotherapy, etc., with some therapeutic progress having been made in recent years, such as neoadjuvant treatment, minimally invasive surgery, modern targeted drugs and precise radiotherapy (4). Unfortunately, the overall treatment efficacy of EC is still unsatisfactory, and as a result, the 5-year survival rate is about 30% (5).
Of all malignant tumors, EC patients have the highest incidence of malnutrition (65–85%) because of insufficient food intake due to esophageal obstruction and tumor consumption (6,7). In addition, the side effects of chemoradiotherapy also increase the risk of malnutrition in patients with EC (8,9). Recent research has indicated that malnutrition is an important factor in the progress and quality of life in patients with EC (10). During treatment, active nutritional support can improve the therapeutic efficacy and reduce the incidence of complications (11). At present, there are many types of nutritional support, of which enteral nutrition (EN) is the first choice.
The Ivor-Lewis esophagectomy uses thoracic and abdominal approaches to replace the esophagus with a tubular stomach, and is a classic operation for the treatment for EC (12,13). Because of its minimal invasiveness and safety, combined with the progress in endoscopic technology over recent years, this surgical treatment is being clinically applied more widely. Prompt EN support is beneficial for patients with EC to improve their nutritional status, sustain immune function, promote postoperative recovery, and reduce the incidence of pulmonary infection, anastomotic leakage, delayed gastric emptying (DGE) and other complications (14,15). However, to date there is not a unified standard for nutritional support after Ivor-Lewis surgery in patients with EC.
The aim of fast track surgery, or enhanced recovery after surgery (ERAS), is to achieve rapid recovery by reducing surgical stress, perioperative organ dysfunction, postoperative complications and shortening hospital stay (16). ERAS aims to establish rapid rehabilitation of complete oral feeding on the first day after operation, with the goal of achieving good clinical benefits (17). However, clinical application of EN support combined with ERAS for EC is still low, so we investigated the effect of EN support combined with ERAS on the nutritional status, immune functional recovery and prognosis of patients with EC after Ivor-Lewis operation.
We present the following article in accordance with the CONSORT reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-3410).
Methods
Inclusion and exclusion criteria
The Ethics Committee of the Second People’s Hospital of Taizhou City approved the present study. 100 patients with EC were enrolled, and written informed consent was given by all the individuals prior to their participation. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Inclusion criteria: (I) patients with EC undergoing thoracoscopic-laparoscopic or laparoscopic right thoracic Ivor-Lewis operation; (II) aged 18–75 years; (III) Karnofsky Performance Score ≥70 before grouping; (IV) patient-generated subjective global assessment ≥2 before grouping; (V) the functioning of heart, lung, liver, spinal cord and other important organs and tissues was essentially normal; (VI) nutritional status or nutritional risk score indicated malnutrition or nutritional risk before grouping; and (VII) able to voluntarily give informed consent and complete the study.
Exclusion criteria: (I) severely impaired gastrointestinal function unable to be supported by EN; (II) EN intolerance, (III) severe digestive tract obstruction, or unable to eat by mouth, or unable to be treated with EN; (IV) severe malnutrition before grouping; and (V) unable to cooperate with the research.
Baseline information
Based on the inclusion and exclusion criteria, 100 patients with EC were randomly divided into an observation group (n=42) and a control group (n=58) using a random number table. The baseline information of all enrolled patients is presented in Table 1. There were no significant differences in the baseline characteristics of the two groups (P>0.05).
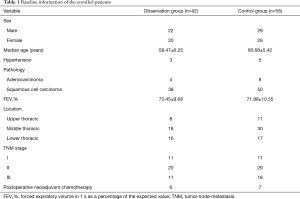
Full table
Protocol
Patients in both groups underwent strict preoperative preparation, including lung function exercises, cough exercises, aspiration prevention and other related clinical education, routine preoperative fasting and water-deprivation, and preoperative enema. A gastrointestinal decompression tube was inserted in the morning of the operation. Thoracoscopic-laparoscopic Ivor-Lewis operation or laparoscopic right thoracic Ivor-Lewis operation was performed under general anesthesia by the same surgical team.
EN management was standardized, including nutritional risk screening, nutritional status assessment, formulation and implementation of optimized nutritional formula, monitoring of nutritional treatment efficacy, dynamic adjustment of the EN program and quality control of the whole process. From postoperative recovery to discharge, patients were guided daily to perform respiratory function exercises, expectoration and coughing to prevent aspiration. The discharge criteria were: (I) no abnormality in vital signs; (II) effective pain relief by oral analgesics; (III) semi-fluid oral intake; (IV) no need for intravenous fluid infusion; (V) free movement; (VI) no exudation, infection or dehiscence of the wound; and (VII) patient’s request to be discharged.
In the observation group, patients were treated with EN combined with ERAS intervention. Cough, expectoration and respiratory function exercises were performed in bed on the first day after operation. Patients were instructed to support their incision to reduce the impact on the anastomotic stoma when coughing. When ultrasound confirmed acceptable lung expansion, and drainage was less than 300 mL/day, the chest tube was removed. If there was no dysuria or obvious prostatic hypertrophy, the urinary catheter was removed. After upper gastrointestinal angiography confirmed that the anastomotic stoma was unobstructed and pyloric emptying was normal, the patients were fed with oral liquid food combined with intravenous nutrition under the guidance of a nutritionist. They were instructed or helped to get out of bed to promote recovery of gastrointestinal function, and the number of times of exercises were performed out of bed was not less than 4 times/day. Patients did not eat for 2 hours before going to sleep and the head of the bed was raised to 30 degrees to prevent aspiration. At 2–3 days after surgery, they continued to eat by mouth under the guidance of the nutritionist, and out of bed exercise sessions were increased to 6–8 times/day. At 4–6 days after the operation, following cessation of intravenous nutrition, the diet was changed to semi-liquid food under the guidance of the nutritionist, and ambulation was increased to 8–10 times/day. At 7 days after the operation, the diet was changed from semi-liquid food to general diet under nutritional guidance.
The patients in control group were treated with a conventional postoperative EN intervention. The preoperative preparation and operative techniques were same as for the observation group. At 1–2 days after surgery, following routine fasting and water-deprivation, the patients were given EN combined with intravenous nutrition. The urethral catheter was removed on the first day after operation, and the chest tube was removed according to the relevant indications. The patients were guided to cough and expectorate to prevent aspiration. At 7 days after operation, upper gastrointestinal angiography was performed to confirm that the anastomotic stoma was unobstructed and there was no disturbance of gastric emptying, after which the gastric tube for oral feeding was removed. At 7–14 days after operation, the intravenous nutrition was stopped. Under the guidance of a nutritionist, the patient was instructed to transit from liquid food to semi liquid food. And patients were advised to cough and expectorate effectively to prevent aspiration until meeting the discharge criteria.
Observation indices
(I) Surgery: operation time, intraoperative blood loss and postoperative chest tube removal time (from end of operation to postoperative chest tube removal), and oral feeding time after operation (from end of operation to implementation of oral feeding). (II) Nutritional biochemistry index: fasting venous blood samples from all of the enrolled patients were collected in the morning of surgery and at discharge. The serum was collected after the samples were centrifuged for 5 min at 1,000 g. The albumin (ALB), transferrin (TF), pre-albumin (PA) and hemoglobin (Hb) levels in serum was measured by automatic blood chemistry analyzer AU5800 (Beckman Coulter, Kraemer, CA, USA). (III) Immune function: immunoturbidimetry was used to assess the tumor immunity of both groups [immunoglobulin (Ig) A, IgG and IgM]. Flow cytometry was used to measure the numbers of CD3+, CD4+ and CD4+/CD8+ T cells. (IV) Prognosis: postoperative complications, including pulmonary infection, incision infection, pleural effusion, DGE, etc., to discharge were recorded. Postoperative recovery, including postoperative exhaust time, postoperative defecation time, postoperative hospital stay and postoperative hospitalization expenses were all recorded.
Statistical analysis
SPSS21.0 software was used to analyze the data. The measured data were tested for normal distribution by Shapiro-Wilk test and for homogeneity of variance by Levene’s test. The data are presented as mean ± standard deviation (SD). Student’s t test was used to analyze the difference between groups. Count data are presented as percentage, and the Chi-square test was performed. Statistical significance for all analyses was accepted at a level of P<0.05.
Results
Comparison of Surgery
Operation time, intraoperative blood loss, postoperative chest tube removal time, and postoperative oral feeding time were compared between the two groups. There was no significant difference in operation time or blood loss between the two groups (P>0.05), but the timing of chest tube removal and oral feeding implementation in the observation group was shorter than in the control group (P<0.05; Table 2).
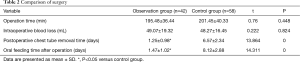
Full table
Comparison of nutritional status
Before the intervention, there were no significant differences in ALB, TF, PA and Hb between the two groups (P>0.05), but in both groups the levels of ALB, TF, PA and Hb were significantly lower before the intervention, and after the intervention the levels in the observation group were significantly higher than those in the control group (P<0.05; Table 3).

Full table
Comparison of immune function
First, humoral immunity was compared. Before the intervention, there was no significant difference in IgA, IgM and IgG levels between the two groups (P>0.05). After the intervention, there was also no significant difference in humoral immune function in the observation group (P>0.05), compared with before intervention, but the IgA, IgM and IgG levels in the control group were significantly decreased (P<0.05), compared with before the intervention. The IgA, IgM and IgG levels in the observation group was significantly higher (P<0.05), compared with the control group after intervention. These results are shown in Table 4.

Full table
Second, the cell immunity was compared. Before the intervention, there was no significant difference in the numbers of CD3+, CD4+ and CD4+/CD8+ T cells between the two groups (P>0.05). After the intervention, there was also no significant difference in the observation group (P>0.05), compared with before the intervention. However, in the control group all T cells were significantly decreased (P<0.05), compared with before intervention. In addition, the numbers of cells in the observation group were significantly higher (P<0.05), compared with the control group after intervention. These results are shown in Table 5.

Full table
Comparison of prognosis
The incidence of pulmonary infection in the observation group was significantly lower than in the control group (P<0.05; Table 6). The postoperative exhaust time, postoperative defecation time and postoperative hospital stay in the observation group were all significantly shorter than in the control group (P<0.05; Table 7). There was no significant difference in hospitalization expenses between the two groups (P>0.05).
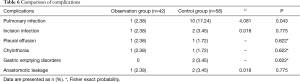
Full table

Full table
Discussion
ERAS has the goal of achieving rapid postoperative recovery by reducing surgical stress, perioperative organ dysfunction, postoperative complications and shortening hospital stay (18,19). ERAS has been widely applied in the postoperative period of gastrointestinal cancers, such as gastric cancer and colorectal cancer (20). The risk of esophagectomy is high, with reported perioperative mortality and respiratory and circulatory complications of 8% and 60% respectively (21,22). The high risk of surgery is an important reason why cases of ERAS in EC are still few. Patients with EC were usually affected by dysphagia, tumor consumption and other factors, leading to them suffering from general malnutrition before treatment (6,23). Combined with the surgical stress response and fasting required for the Ivor-Lewis operation, postoperative immune dysfunction is very likely, and the risk of postoperative complications is high, even life-threatening. Many studies have confirmed that the nutritional status of patients with EC is closely related to their prognosis (24), so it is very important to improve it.
For patients with EC, EN has significant advantages in reducing various complications of the cancer, because it effectively nourishes the intestinal mucosa, maintains the intestinal barrier, and is more conducive to postoperative recovery (7). However, to date there is no uniform standard for the timing and mode of EN for patients with EC. Previous studies considered that due to the effect of surgery, patients with EC had intestinal paralysis for 3 days after surgery, which led to gastrointestinal disorders (1,25). However, some studies have confirmed that intestinal absorption and peristalsis can be restored 6 h after operation, and suggested that EN should be actively carried out when the intestinal tract is functional and safe (6,26). EN is an important part of ERAS, and influences the complete implementation of the ERAS process (27). Differences exist in the type of EN and its timing during combined EN and ERAS, and reports on combined EN support and ERAS after the Ivor-Lewis operation for EC are few.
In our study, there were no significant differences in operation time and blood loss between the two groups (P>0.05), but the timing of both chest tube removal and oral feeding in the observation group was shorter than in the control group (P<0.05). The ERAS intervention significantly shortened the time of chest tube removal and oral feeding after Ivor-Lewis operation for EC. The levels of ALB, TF, PA and Hb in the two groups were significantly lower than before treatment, but the levels of ALB, TF, PA and Hb in the observation group were significantly higher than those in the control group (P<0.05). These results indicated that both groups showed some nutritional loss, but it was less in the observation group than in the control group. Effective, scientific and appropriate EN can improve the perioperative nutritional loss of patients with EC, and it is better to institute EN early.
Intestinal flora are an important part of the intestinal mucosal barrier, and disturbance of the intestinal flora can increase the risk of perioperative complications (28,29). Gastrointestinal surgery will destroy the intestinal mucosal barrier and increase the risk of postoperative pulmonary infection, anastomotic leakage and other complications (30). Some research has also confirmed that the cell-mediated immune response was impaired, the numbers of CD4+ and CD4+/CD8+ T cells decreased, and the intestinal barrier function was damaged in patients with EC after laparoscopic Ivor-Lewis surgery (31). How to improve the immune response is the key to preventing these complications. Studies have confirmed that nutritional status is associated with immune function, and malnutrition can lead to some loss of immune function. We showed that there were no significant changes in IgA, IgG and IgM, or in CD3+, CD4+ and CD4+/CD8+ T cells in the observation group before and after intervention (P>0.05), but all these indexes in the control group were significantly lower after intervention than before intervention (P<0.05). More important, the values in the observation group were all higher than those in the control group (P<0.05). These results indicated that the immune status of patients with EC after Ivor-Lewis operation was decreased, but that EN combined with ERAS could maintain relatively good immune status. In addition, the incidence of pulmonary infection, the postoperative exhaust time, postoperative defecation time and postoperative hospital stay in the observation group were all significantly less than in the control group (P<0.05). The shortening of postoperative exhaust time and postoperative defecation time were related to the application of ERAS. However, there was no significant difference in hospitalization expenses between the two groups.
In summary, EN support combined with EARS was beneficial for improving the nutritional status and immune function recovery of patients with EC after Ivor-Lewis operation. It also contributed to shortening the length of hospital stay and improving the short-term prognosis of patients with EC after operation.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-3410
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-3410
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-3410). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Ethics Committee of the Second People’s Hospital of Taizhou City approved the present study. 100 patients with EC were enrolled, and written informed consent was given by all the individuals prior to their participation. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kelly RJ. Emerging multimodality approaches to treat localized esophageal cancer. J Natl Compr Canc Netw 2019;17:1009-14. [Crossref] [PubMed]
- Tang WR, Chen ZJ, Lin K, et al. Development of esophageal cancer in Chaoshan region, China: association with environmental, genetic and cultural factors. Int J Hyg Environ Health 2015;218:12-8. [Crossref] [PubMed]
- Liu Q, Zeng H, Xia R, et al. Health-related quality of life of esophageal cancer patients in daily life after treatment: A multicenter cross-sectional study in China. Cancer Med 2018;7:5803-11. [Crossref] [PubMed]
- Mönig S, Chevallay M, Niclauss N, et al. Early esophageal cancer: the significance of surgery, endoscopy, and chemoradiation. Ann N Y Acad Sci 2018;1434:115-23. [Crossref] [PubMed]
- Dong J, Gu X, El-Serag HB, et al. Underuse of Surgery Accounts for Racial Disparities in Esophageal Cancer Survival Times: A Matched Cohort Study. Clin Gastroenterol Hepatol 2019;17:657-665.e13. [Crossref] [PubMed]
- Steenhagen E, van Vulpen JK, van Hillegersberg R, et al. Nutrition in peri-operative esophageal cancer management. Expert Rev Gastroenterol Hepatol 2017;11:663-72. [Crossref] [PubMed]
- Watanabe M, Okamura A, Toihata T, et al. Recent progress in perioperative management of patients undergoing esophagectomy for esophageal cancer. Esophagus 2018;15:160-4. [Crossref] [PubMed]
- Kanekiyo S, Takeda S, Iida M, et al. Efficacy of perioperative immunonutrition in esophageal cancer patients undergoing esophagectomy. Nutrition 2019;59:96-102. [Crossref] [PubMed]
- Kempf E, Tournigand C, Rochigneux P, et al. Discrepancies in the use of chemotherapy and artificial nutrition near the end of life for hospitalised patients with metastatic gastric or oesophageal cancer. A countrywide, register-based study. Eur J Cancer 2017;79:31-40. [Crossref] [PubMed]
- Mansfield SA, El-Dika S, Krishna SG, et al. Routine staging with endoscopic ultrasound in patients with obstructing esophageal cancer and dysphagia rarely impacts treatment decisions. Surg Endosc 2017;31:3227-33. [Crossref] [PubMed]
- Qiu ML, Lin JB, Li X, et al. Current state of esophageal cancer surgery in China: a national database analysis. BMC Cancer 2019;19:1064. [Crossref] [PubMed]
- Takahashi K, Mine S, Kozuki R, et al. Ivor-Lewis esophagectomy for patients with squamous cell carcinoma of the thoracic esophagus with a history of total pharyngolaryngectomy. Esophagus 2019;16:382-5. [Crossref] [PubMed]
- Shi H, Han Y, Peng L. Sweet Versus Ivor-Lewis: Is It Time To Draw a Conclusion? Ann Surg 2018;268:e34-e35. [Crossref] [PubMed]
- Ma L, Luo GY, Ren YF, et al. Concurrent chemoradiotherapy combined with enteral nutrition support: a radical treatment strategy for esophageal squamous cell carcinoma patients with malignant fistulae. Chin J Cancer 2017;36:8. [Crossref] [PubMed]
- Wang B, Jiang X, Tian D, et al. Enteral nutritional support in patients undergoing chemoradiotherapy for esophageal carcinoma. Future Oncol 2020;16:2949-57. [Crossref] [PubMed]
- Grant MC, Yang D, Wu CL, et al. Impact of enhanced recovery after surgery and fast track surgery pathways on healthcare-associated infections: results from a systematic review and meta-analysis. Ann Surg 2017;265:68-79. [Crossref] [PubMed]
- Rubinkiewicz M, Witowski J, Su M, et al. Enhanced recovery after surgery (ERAS) programs for esophagectomy. J Thorac Dis 2019;11:S685-S691. [Crossref] [PubMed]
- Vannucci J, Costi S, Matricardi A, et al. VATS Group ERAS Registry. J Thorac Dis 2018;10:S571-S577. [Crossref] [PubMed]
- Schneider S, Armbrust R, Spies C, et al. Prehabilitation programs and ERAS protocols in gynecological oncology: a comprehensive review. Arch Gynecol Obstet 2020;301:315-26. [Crossref] [PubMed]
- Zhang Y, Xin Y, Sun P, et al. Factors associated with failure of Enhanced Recovery After Surgery (ERAS) in colorectal and gastric surgery. Scand J Gastroenterol 2019;54:1124-31. [Crossref] [PubMed]
- van der Horst S, de Maat MFG, van der Sluis PC, et al. Extended thoracic lymph node dissection in robotic-assisted minimal invasive esophagectomy (RAMIE) for patients with superior mediastinal lymph node metastasis. Ann Cardiothorac Surg 2019;8:218-25. [Crossref] [PubMed]
- Valsangkar N, Salfity HVN, Timsina L, et al. Operative time in esophagectomy: Does it affect outcomes? Surgery 2018;164:866-71. [Crossref] [PubMed]
- Zhou XL, Zhu WG, Zhu ZJ, et al. Lymphopenia in Esophageal Squamous Cell Carcinoma: Relationship to Malnutrition, Various Disease Parameters, and Response to Concurrent Chemoradiotherapy. Oncologist 2019;24:e677-e686. [Crossref] [PubMed]
- Dupont R, Longue M, Galinier A, et al. Impact of micronutrient deficiency & malnutrition in systemic sclerosis: Cohort study and literature review. Autoimmun Rev 2018;17:1081-9. [Crossref] [PubMed]
- Batra R, Malhotra GK, Singh S, et al. Managing Squamous Cell Esophageal Cancer. Surg Clin North Am 2019;99:529-41. [Crossref] [PubMed]
- Lahoud J, Bazzi K, Yeo D, et al. Survey of nutritional practices in total gastrectomy and oesophagectomy procedures. Nutr Diet 2019;76:135-40. [Crossref] [PubMed]
- Medbery RL, Fernandez FG, Khullar OV. ERAS and patient reported outcomes in thoracic surgery: a review of current data. J Thorac Dis 2019;11:S976-S986. [Crossref] [PubMed]
- Wardill HR, da Silva Ferreira AR, Lichtenberg Cloo S, et al. Pre-therapy fasting slows epithelial turnover and modulates the microbiota but fails to mitigate methotrexate-induced gastrointestinal mucositis. Gut Microbes 2020;12:1-9. [Crossref] [PubMed]
- Ames NJ, Barb JJ, Schuebel K, et al. Longitudinal gut microbiome changes in alcohol use disorder are influenced by abstinence and drinking quantity. Gut Microbes 2020;11:1608-31. [Crossref] [PubMed]
- Zhou LY, Deng MQ, Xiao XH. Potential contribution of the gut microbiota to hypoglycemia after gastric bypass surgery. Chin Med J (Engl) 2020;133:1834-43. [Crossref] [PubMed]
- Wheeler JC, Vanoni S, Zeng C, et al. 17beta-Estradiol protects the esophageal epithelium from IL-13-induced barrier dysfunction and remodeling. J Allergy Clin Immunol 2019;143:2131-46. [Crossref] [PubMed]
(English Language Editor: K. Brown)


