Surgical staging and resection of malignant pleural mesothelioma
Introduction
Mesothelioma is a malignancy of mesothelial cells, which line the pleura, peritoneum, pericardium, and tunica vaginalis. Malignancy of the pleural lining is most commonly associated with occupational exposure to asbestos (1). Mesothelioma is four times more likely to present in men than in women due to workplace exposures, and the median age of diagnosis is 74. Typically, there is a 20- to 50-year delay between asbestos exposure and the development of mesothelioma (2).
Mesothelioma represents less than 1% of all cancers, but it has a particularly poor prognosis (1). Approximately 3,200 people are diagnosed with mesothelioma every year in the United States (US) (3). Patients often present with wheezing or hoarseness, shortness of breath, a persistent cough, and pain in the chest or back. Many of these patients are diagnosed with advanced disease, and the five-year relative survival rate is 10% (2).
Staging and subtyping are the first and most important steps in determining which treatment is best for an individual patient. An expert pathologist from an experienced medical center should review all relevant tissue biopsies. Evaluation from a needle thoracentesis may not prove to be adequate for staging purposes, as effusion fluid cytology does not allow assessment of invasion (4).
When a patient has a history of significant exposure to asbestos, shipyards, or construction, along with a pleural effusion, the suspicion for mesothelioma should arise. This suspicion will cause the thoracic interventionalist to approach the drainage and evaluation differently than if this were a known other metastatic tumor or benign pleural effusion pleural effusion.
Staging and operative techniques
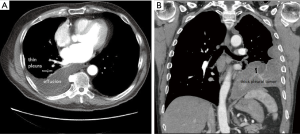
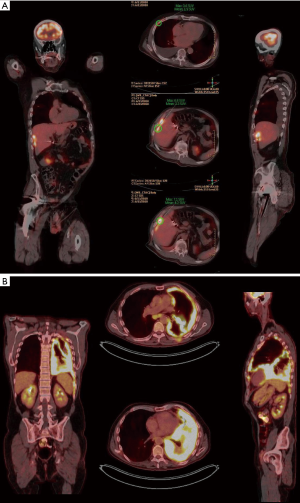
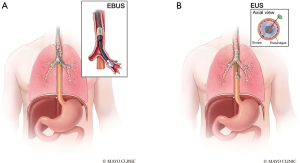
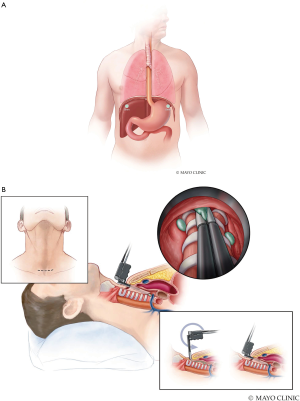
Proper staging involves a combination of imaging and surgical evaluation. Computed tomography (CT) (Figure 1A,B) and positron emission tomography (PET) (Figure 2A,B) scans can delineate mediastinal involvement with short axis diameter greater than 1cm or increased FDG activity. Endobronchial ultrasound (EBUS) (Figure 3A) or endoscopic ultrasound (EUS) (Figure 3B) can often confirm pathology without requiring the patient to undergo a surgical staging mediastinoscopy (5). When metastatic disease is suspected, a needle core biopsy can confirm the presence of mesothelial malignant cells in tissue (6). There are three basic surgical staging procedures that are required to assess a patient’s candidacy for surgical intervention and complete staging when no clear imaging indicates they have metastatic disease; mediastinoscopy, laparoscopy, and thoracoscopy.
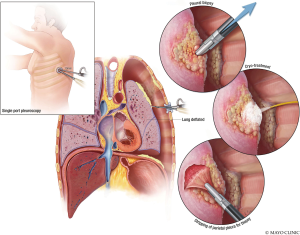
The three minimally invasive procedures are delineated in this technique illustration. The first technique includes a thoracoscopic biopsy and evaluation of the pleura (Figure 4). Once the tissue type is confirmed, the surgeon may desire to proceed with laparoscopic abdominal evaluation (Figure 5A) and mediastinoscopy (Figure 5B) (5). Performing these procedures in the correct order will allow the patient to avoid unnecessary surgery. Once staging has been performed, the findings should be reviewed with the patient for an open discussion about expectations, findings, and which treatment may be best for them based on their goals of care (Table 1). Patients should be educated about disease stage prior to performing irreversible procedures such as talc, resection, or therapy.

Full table
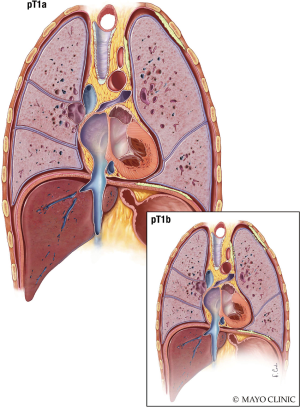
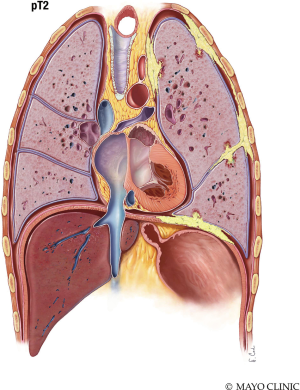
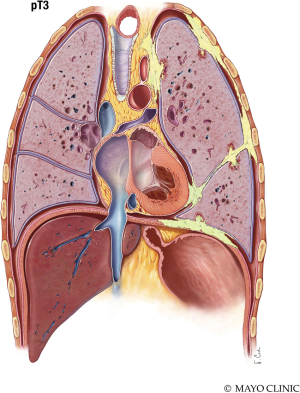
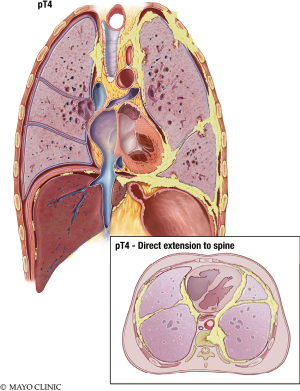
The T stage describes the stage characteristics of the tumor. T1 Mesothelioma (Figure 6) is limited to the ipsilateral parietal pleura, with or without involvement of the visceral pleura. The tumor may also include the diaphragmatic and mediastinal pleural. T2 Mesothelioma (Figure 7) involves the ipsilateral pleura surfaces (parietal, diaphragmatic, mediastinal, and visceral pleura) with at least one of the following: extension into the lung parenchyma, invasion of the diaphragmatic muscle, and/or a confluent visceral pleura tumor (including the fissure). T3 Mesothelioma (Figure 8) involves any ipsilateral pleural surfaces, with at least one of the following: extension into mediastinal fat, invasion of the endo-thoracic fascia, non-transmural involvement of the pericardium, and/or a solitary focus of tumor invading the soft tissues of the chest wall. T4 Mesothelioma (Figure 9) is characterized by diffuse extension or multifocal masses of tumor in the chest wall, with potential rib destruction. Additionally, the tumor may extend into the contralateral pleura, spine, mediastinal organs, through the internal surface of the pericardium (with or without a pericardial effusion; or tumor involving the myocardium), and/or trans-diaphragmatic into the peritoneum (Table 2) (7,8).

Full table
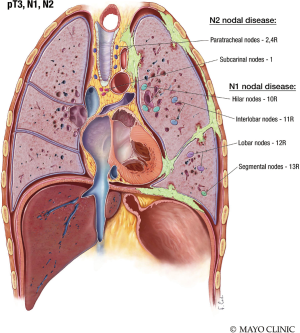
The N stage describes the nodal stage. N1 nodal disease is characterized by metastasis in the ipsilateral bronchopulmonary, mediastinal, or hilar lymph nodes. N2 nodal disease spreads to the contralateral mediastinal, subcarinal or ipsilateral paratracheal lymph nodes, or ipsilateral or contralateral supraclavicular nodes (Figure 10) (7,8).
The M stage is related to metastasis. M0 demonstrates no distant metastasis and M1 demonstrates that metastasis is present (Table 2) (7,8).
Staging (IA–IV) for malignant pleural mesothelioma (MPM) is related to the patient’s TNM characteristics (Table 3).
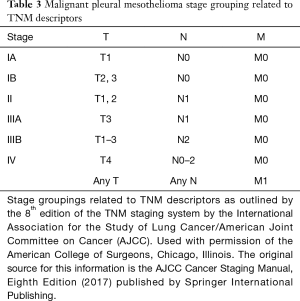
Full table
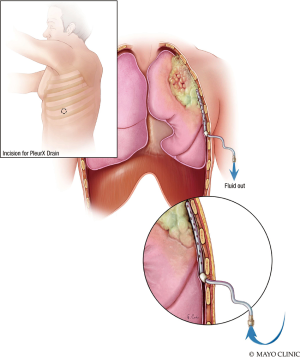
Insertion of a PleurX catheter is safe and effective to provide palliation for recurrent malignant pleural effusions caused by Mesothelioma (Figure 11) (9). This tunneled indwelling pleural catheter is inserted percutaneously and allows for intermittent drainage of effusions outside of the hospital setting (10). Alternatively, talc pleurodesis or other pleurodesis technology may be employed to relieve a patient from dyspnea due to recurrent effusions. It is important talc not be instilled until the patient has been evaluated by an expert mesothelioma care team, staged, offered enrollment into clinical research, discussed surgical options with a surgeon expert in the field, and has been given an opportunity to decide what pathway they choose, as placing talc inside the chest of a mesothelioma patient can burn bridges for therapy.
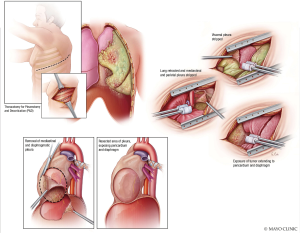
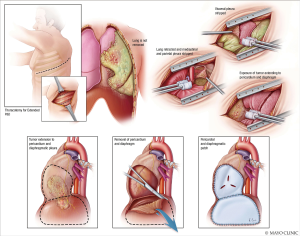
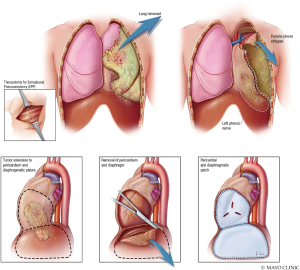
Pleurectomy and decortication (P/D) (Figure 12) is one of the two surgical procedures recommended for MPM. It involves thoracotomy, extrapleural dissection of the parietal and visceral pleura, and mediastinal lymph node dissection. Resection of the diaphragm and/or pericardium may be necessary if these structures are invaded (11). P/D (Figure 13) may include reconstruction of the diaphragm and pericardium with expanded polytetrafluoroethylene (ePTFE) mesh. ePTFE is an inert polymer of monofilament threads that will integrate completely with the patient in seven months (12). Extrapleural pneumonectomy (EPP) (Figure 14) goes a step further than P/D, and involves en bloc resection of the lung, pleura, pericardium, and diaphragm (11). For either pleurectomy and decortication or extra-pleural pneumonectomy, the surgeon often has to make a 7th intercostal space incision for thoracotomy, but then enter through the 4–6th intercostal space and 7–9th intercostal space to provide enough access to complete the incision. This is a single incision, double intercostal space thoracotomy. Often, epidural coverage may be difficult. This surgery is often painful and has associated morbidity.
Surgical eligibility and outcomes
The choice of surgical extirpation for MPM should be determined by a multi-disciplinary team, including a surgeon. The role of surgery is for diagnosis, staging, and macroscopic resection of disease; surgery should be reserved for patients with good performance status and with stage I–III disease. Patients with a sarcomatoid histology (the most aggressive mesothelioma cell type) or stage IV MPM should not undergo a surgical procedure, unless for a palliative reason (13).
P/D should be considered the first choice of treatment for patients with early stage disease (stage I, N0-1). On the other hand, while EPP often has an increased morbidity and mortality when compared to P/D, this technique may permit a more complete resection in those patients identified to have bulky deep fissure involvement (13,14).
Morbidity and mortality are different for EPP, as compared to P/D. Patients who undergo resection of the lung, hemidiaphragm, and ipsilateral pericardium (during an EPP) may experience additional hemodynamic and cardiorespiratory complications postoperatively. The most common complication following a P/D is a prolonged air leak, associated with trauma to the lung tissue during removal of the pleura (13,14).
Mortality in EPP usually results from bronchopleural fistula (leading to sepsis and multiorgan failure) and pulmonary embolism. However, in P/D mortality was usually the result of atelectasis, pneumonia, air leak, localized infection, pulmonary embolism, and empyema. While P/D may have a lower perioperative morbidity and mortality, long-term cure among those surviving may be slightly lower when compared to survivors of EPP (13,14).
Patients who undergo EPP should be able to tolerate a pneumonectomy and should have a pre-operative testing to determine their predicted postoperative forced expired volume in the first second of expiration (FEV1) and diffusing capacity of the lung for carbon monoxide (DLCO). Patients with predicted post-operative values greater than 40% are considered candidates for pneumonectomy. However, pre-operative values between 30–60% of predicted required further work-up prior to surgery, and less than 30% post-operative values are considered to be high-risk candidates (15).
Most patients are not cured of their disease after resection, although there are some patients who may experience long-term survival or cure. However, the combination of therapies (both surgical and nonsurgical) are typically administered with curative intent (13,14). We recommend that eligible patients are enrolled into clinical trials in an effort to prospectively answer questions and improve care in such a rare disease.
Adjunctive surgical therapy
Other therapies like cryotherapy, heated intrapleural chemotherapy, photodynamic therapy, or intraoperative radiation can be employed in conjunction with surgical resection (16,17). However, the addition of neoadjuvant or adjuvant chemotherapy and radiation in sequence with surgery is typically made in a multi-disciplinary tumor board after complete staging is performed.
Pitfalls/comparisons
Due to a poor prognosis, the management of pleural mesothelioma remains controversial. Many of the early staging systems reflected institutional-level experiences and were not externally validated. Presently, the classification outlined by the International Mesothelioma Interest Group (IMIG), the International Association for the Study of Lung Cancer (IASLC), and the American Joint Committee on Cancer (AJCC), have been adopted for clinical use. These recommendations consider surgical and pathologic variables as well as cross-sectional imaging (5,7).
Pearls
Single port video-assisted thoracoscopy (VATS) is a reliable method to diagnose intrathoracic conditions. A single incision from 0.5 to 2.5 cm in length is made, followed by blunt dissection to the pleural plane and introduction of articulating operative instruments parallel to a 5-mm 0° or 30° video-thoracoscope allows access both for diagnosis and evaluation for staging without creating too many ports that need to be later excised or are seeding for extrapleural spread of tumor (18). Placement of the incision is determined by the location of the intrathoracic pathology as well as where a thoracotomy may be later placed to include this incision for removal, and, thus careful review of the chest computed tomography is crucial. Unlike, traditional VATS, the pleural evaluation may also be performed safely under locoregional anesthesia (through an epidural catheter) and without intubation (medical pleuroscopy). When performing the surgical removal of mesothelioma, the surgeon may choose to make one external thoracotomy incision but then two separate intercostal incisions so that the entire chest may be reached. Additionally, placing a laparoscopic camera into the open thoracotomy wound during resection may provide additional access into the chest with enhanced visualization for learners who might be in the room as well as improved resection from an enhanced view of the intrathoracic cavity.
Acknowledgments
We would like to thank and acknowledge the media services of Mayo Clinic for their assistance in creating the included illustrations. All images are used with permission of Mayo Foundation for Medical Education and Research, all rights reserved.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-19-2267). SHB reports grants from Steris and Medtronic, outside the submitted work. These funds are only used to fund research. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All of the included figures were created by a medical illustrator working with our team, and no human subjects were used in the creation of this project. However, our team understands that all procedures performed in studies involving human participants should be in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Moore AJ, Parker RJ, Wiggins J. Malignant mesothelioma. Orphanet J Rare Dis 2008;3:34. [Crossref] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2017 [Internet]. American Cancer Society. 2017. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf
- Henley SJ, Larson TC, Wu M, et al. Mesothelioma incidence in 50 states and the District of Columbia, United States, 2003-2008. Int J Occup Environ Health 2013;19:1-10. [Crossref] [PubMed]
- van Zandwijk N, Clarke C, Henderson D, et al. Guidelines for the diagnosis and treatment of malignant pleural mesothelioma. J Thorac Dis 2013;5:E254-E307. [PubMed]
- Bonomi M, De Filippis C, Lopci E, et al. Clinical staging of malignant pleural mesothelioma: current perspectives. Lung Cancer (Auckl) 2017;8:127-39. [Crossref] [PubMed]
- Metintaş M, Ozdemir N, Işiksoy S, et al. CT-guided pleural needle biopsy in the diagnosis of malignant mesothelioma. J Comput Assist Tomogr 1995;19:370-4. [Crossref] [PubMed]
- Amin MB, Edge S, Greene F, et al. AJCC Cancer Staging Manual Eighth edition. Springer Int Publ, 2017.
- Rusch VW. A proposed new international TNM staging system for malignant pleural mesothelioma. From the International Mesothelioma Interest Group. Chest 1995;108:1122-8. [Crossref] [PubMed]
- Meier M, Mortensen MR, Larsen LU. Implantation of permanent pleural catheter for palliation of malignant pleural effusion. Cancer Manag Res 2016;8:129-33. [Crossref] [PubMed]
- Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med 2011;26:70-6. [Crossref] [PubMed]
- Hasegawa S. Extrapleural pneumonectomy or pleurectomy/decortication for malignant pleural mesothelioma. Gen Thorac Cardiovasc Surg 2014;62:516-21. [Crossref] [PubMed]
- Solli P, Brandolini J, Pardolesi A, et al. Diaphragmatic and pericardial reconstruction after surgery for malignant pleural mesothelioma. J Thorac Dis 2018;10:S298-303. [Crossref] [PubMed]
- Ricciardi S, Cardillo G, Zirafa CC, et al. Surgery for malignant pleural mesothelioma: an international guidelines review. J Thorac Dis 2018;10:S285-92. [Crossref] [PubMed]
- Batirel HF. Extrapleural pneumonectomy (EPP) vs. pleurectomy decortication (P/D). Ann Transl Med 2017;5:232. [Crossref] [PubMed]
- Roy PM. Preoperative pulmonary evaluation for lung resection. J Anaesthesiol Clin Pharmacol 2018;34:296-300. [PubMed]
- Cramer G, Simone CB 2nd, Busch TM, et al. Adjuvant, neoadjuvant, and definitive radiation therapy for malignant pleural mesothelioma. J Thorac Dis 2018;10:S2565-S2573. [Crossref] [PubMed]
- Rosenzweig KE. Malignant pleural mesothelioma: adjuvant therapy with radiation therapy. Ann Transl Med 2017;5:242. [Crossref] [PubMed]
- Rocco G. One-port (uniportal) video-assisted thoracic surgical resections--a clear advance. J Thorac Cardiovasc Surg 2012;144:S27-31. [Crossref] [PubMed]