
Uniport vs. multiport video-assisted thoracoscopic surgery for anatomical lung resection—which is less invasive?
Introduction
Video-assisted thoracoscopic surgery (VATS) has proven to be a safe and effective surgical procedure that has been widely practiced; uniport VATS (U-VATS) has been pursued in search of a more minimally invasive surgical procedure. U-VATS was first reported by Rocco in 2004 for wedge resection (1), Gonzalez-Rivas later reported anatomical lung resection with U-VATS (2,3), and it has developed to more complex surgery, such as segmentectomy and sleeve resection (4). We started U-VATS in February 2019 and have performed more than 80 anatomical lung resections so far. In U-VATS, we use special curved forceps and a suction tube, and we have devised a length and angle so that the instruments do not interfere with each other.
On the other hand, whether U-VATS is truly minimally invasive for patients needs to be determined. The long-term prognosis needs to be evaluated for the therapeutic effect, but short-term evaluation is necessary to evaluate perioperative outcomes and pain for minimal invasiveness. Thus, the perioperative results of U-VATS were compared with those of conventional multiport VATS (M-VATS), and the postoperative analgesic prescription period was investigated. The aim of this study is to evaluate which approach reduced postoperative pain earlier and was less invasive in anatomical lung resection.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-2759).
Methods
Patient selection
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Maebashi Red Cross Hospital (NO.: 2020-17) and individual consent for this retrospective analysis was waived.
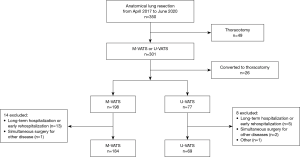
From April 2017 to July 2020, a total of 350 patients underwent anatomical lung resection for malignant or benign lung diseases at our hospital. Data were obtained from the medical records of patients who underwent anatomical lung resection of lesions during the same period. Cases of thoracotomy (n=49) and conversion to thoracotomy (n=26) were excluded. In addition, the following patients were excluded to eliminate factors that affect evaluation of postoperative pain. Fourteen patients in the M-VATS group were excluded due to simultaneous surgery for other disease (n=1), long-term hospitalization (>10 days) due to complications (3 for continuous air leakage, 1 for bleeding, 1 for organizing pneumonia), and early rehospitalization (4 for pleural effusion that required drainage, 3 for pneumothorax that required drainage, 1 for angina). In the same way, eight patients in the U-VATS group were excluded due to simultaneous surgery for other disease (n=2), long-term hospitalization (>10 days) due to complications (1 for cerebral infarction, 1 for organizing pneumonia), and early rehospitalization (2 for pneumothorax that required drainage, 1 for contralateral pneumothorax). Additionally, a 90-year-old man in the U-VATS group died suddenly at home for no known reason 2 days after discharge and was excluded from the study. Finally, 184 patients in the M-VATS group and 69 patients in the U-VATS group were enrolled (Figure 1). M-VATS was performed by three senior surgeons and two surgeons with intermediate experience, and U-VATS was performed by two of the three senior surgeons. The surgical procedure was decided by the surgeon. All patient data were analyzed retrospectively.
Procedure for U-VATS
All surgical procedures were performed with the patient under general anesthesia with double-lumen intubation. The surgeon stood on the ventral side of the patient and the scopist on the dorsal side. A 3.5- to 4.0-cm skin incision was made at the 4th or 5th intercostal space in the anterior axillary line, and an Alexis wound retractor XS (Applied Medical, Rancho Santa Margarita, CA, USA) was attached. The thoracoscope (5- or 10-mm, 30-degree) was fixed to the dorsal wound edge. Basically, endoscopic staplers were used to separate the pulmonary vessels and bronchus, but in cases of segmentectomy, ligation with silk was performed according to the diameter of the vessels. All lung parenchyma was cut with an endoscopic stapler. A drainage tube was placed from the ventral wound edge.
Procedure for M-VATS
M-VATS was performed with 3 or 4 ports. A 2.0-cm skin incision was made in the 4th intercostal space in the anterior axillary line, a 1.5-cm skin incision was made in the 6th intercostal space in the anterior axillary line, and an Alexis wound retractor XXS was attached to each. A 1.5-cm skin incision was made in the posterior axillary line of the sixth intercostal space, and a 10-mm flexible camera was inserted. In the case of 4 ports, a 15-mm skin incision was made under the 7th intercostal space at the scapula and used as an assistant port. The method of lung resection was the same as that in U-VATS, and the drainage tube was placed from the 6th intercostal space in the anterior axillary line port.
Postoperative management
Immediately after the operation, patient-controlled analgesia (PCA) with fentanyl at 0.006 µg/kg/min was used, and further intravenous infusion of acetaminophen was given. When oral administration became possible, oral administration of non-steroidal anti-inflammatory drugs (NSAIDs) was started, and PCA ended on postoperative day 1.
The chest drain was removed after confirming that there was no air leakage and the daily drainage amount was <200 mL. If the patient had major postoperative air leakage on postoperative day 2, pleurodesis to stop it was performed on the same day or afterwards. In our department, OK-432, minocycline, or autoblood was administered into the thoracic cavity via a thoracic drainage tube during the pleurodesis procedure.
The patients could be discharged if the chest X-ray taken the day after chest drain removal did not show any problem. Most patients made their first outpatient visit by the 7th to 10th day postoperatively, and if they complained of pain, additional analgesics were prescribed.
These postoperative management were the same in both groups.
Statistical analysis
Categorical variables were compared using Fisher’s exact test. Continuous variables were compared using the t-test. Multivariate analyses were performed using a logistic regression model. Results were considered significant for values of P<0.05. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics.
Results
Characteristics and clinicopathological features
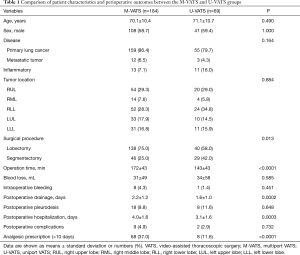
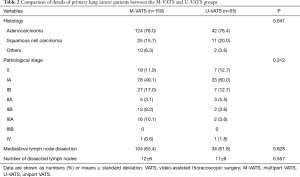
The characteristics and clinicopathological features of all patients are shown in Tables 1,2. There were no significant differences between the groups in age, sex, underlying disease, and tumor location. Among surgical procedures, segmentectomy was significantly more common in the U-VATS group (P=0.013). In primary lung cancer patients, there were no significant differences between the groups in histology, pathological stage, mediastinal lymph node dissection rate, and number of dissected lymph nodes.
Full table
Full table
Perioperative outcomes
Details of perioperative results are shown in Table 1. The mean operation time was significantly shorter in U-VATS than in M-VATS (172±43 min in M-VATS vs. 143±43 min in U-VATS, P<0.0001). There were no significant differences between the groups in intraoperative blood loss and the rate of intraoperative significant bleeding (bleeding from the pulmonary artery or vein that could be managed under VATS). Duration of postoperative drainage was significantly shorter in U-VATS than in M-VATS (2.2±1.2 days in M-VATS vs. 1.6±1.0 days in U-VATS, P=0.0002). The duration of postoperative hospitalization was also significantly shorter in U-VATS than in M-VATS (4.0±1.6 days in M-VATS vs. 3.1±1.6 days in U-VATS, P=0.0003). There were no significant differences between the groups in the rates of postoperative pleurodesis.
Postoperative complications occurred in 9 patients (4.9%) in M-VATS: pneumonitis in 3, prolonged air leak in 2, atrial fibrillation in 2, pneumonitis in 3, chylothorax in 1, and heart failure in 1. On the other hand, postoperative complications occurred in 2 patients (2.9%) in U-VATS: prolonged air leak in 1 and atrial fibrillation in 1. There was no significant difference in the rate of postoperative complications between the groups (P=0.732).
The number of analgesic prescriptions over 10 days postoperatively was significantly less in U-VATS than in M-VATS [68 (37.0%) in M-VATS vs. 8 (11.6%) in U-VATS, P<0.0001].
Multivariate analyses of factors related to analgesic prescription
Postoperative pain may be affected by various factors, such as age, sex, surgical procedure, and so on. Postoperative drainage duration and with or without pleurodesis also have a significant effect. A multivariate analysis of analgesic prescription 10 days postoperatively was performed based on age, sex, U-VATS or M-VATS, drainage duration, operation time, and with or without pleurodesis. The multivariate logistic regression model showed that U-VATS was the only significant predictor (odds ratio =0.204, P=0.0001) (Table 3).

Full table
Discussion
The present study indicated that U-VATS reduced postoperative pain earlier than M-VATS. The multivariate logistic regression model showed that U-VATS was the only significant predictor of reduced postoperative pain. Furthermore, this study showed comparable perioperative results for U-VATS and M-VATS, including shortened operation time, drainage duration, and postoperative hospitalization.
Various studies have tried to reduce postoperative pain by decreasing the number of ports in VATS. Most of them were retrospective, but many centers reported pain relief after U-VATS. In surgery for spontaneous pneumothorax, Yang et al. (5) demonstrated in their systematic review and meta-analysis that single-incision thoracoscopic surgery (SITS) was associated with less postoperative pain, a lower paresthesia rate, and a shorter hospital stay. Nachira et al. (6) demonstrated in their study that compared U-VATS and M-VATS for primary spontaneous pneumothorax that there was a significant difference in favor of U-VATS in the visual analogue scale (VAS) score for pain at 24 h (P<0.001), postoperative pain duration (P<0.001), analgesic intake (P=0.001), chronic paresthesia (P<0.001), and chronic neuralgia (P<0.001). U-VATS was also reported to significantly reduce postoperative pain during anatomical lung resection for lung cancer (7-11). However, these studies were all comparative studies with univariate analyses. Postoperative pain may be affected by various factors, including operation time, drainage duration, and postoperative complications such as a prolonged air leak that requires pleurodesis. In the present study, factors that purely affect postoperative pain were analyzed, excluding cases with long-term drainage, early readmission, and complications of other diseases. This is the first report that examined the causes of postoperative pain by multivariate analysis considering these factors.
In thoracic surgery, patients sometimes complain not only of normal wound pain, but also of paresthesia around the wound (12,13). Maguire et al. (12) performed a questionnaire study about the neuropathic component of chronic pain after thoracic surgery and reported that the prevalence of chronic pain was 57% at 7–12 months and 36% at 4–5 years. However, there was no significant difference by surgical approach (thoracotomy or VATS). During VATS, it is possible that excessive torqueing of the camera or instruments at the intercostal space can injure the intercostal nerve and damage the rib. This likelihood of intercostal neurovascular bundle injury is also potentially increased if proper caution is not exercised when instruments are introduced through the narrower confines of the posterior aspect of the intercostal space (14). In U-VATS, the incision is only in the anterior axillary line with a relatively wide intercostal space, and the absence of a port in the mid or posterior axillary line may prevent intercostal neuropathy on the narrow dorsal side of the intercostal space, leading to pain relief.
In the present study, pain scoring, such as a VAS, and patient satisfaction were not evaluated. In our department, the patient was usually discharged the day after removal of the postoperative drainage tube. Moreover, the postoperative drainage tube strongly affected postoperative pain. Therefore, it was considered difficult to adequately evaluate how the surgical approach including uniport or multiport could affect the postoperative pain during hospitalization. In addition, our team always asked the patients at their first visit to the outpatient clinic whether they needed additional analgesic prescriptions as postoperative pain relief, which was important to provide high postoperative quality of life. Thus, in the present study, postoperative pain was evaluated by comparing the rate of patients requiring analgesic prescriptions over 10 days postoperatively to reduce the postoperative pain between the two groups. We believe this evaluation has validity, objectivity to some extent, and is easy to understand, because whether the analgesic was prescribed or not was obvious in the medical record, although a VAS or NRS is commonly used for scoring.
In the present study, operation time, postoperative drainage duration, and hospitalization were significantly shorter in U-VATS than in M-VATS. The reasons for this operation time shortening are: (I) differences in experience and technology due to the operators who are familiar with M-VATS and have transitioned to U-VATS; (II) the camera’s viewpoint from the anterior axillary line is directly aimed at the operator's target, which has the same field of view as thoracotomy; and (III) U-VATS does not require deployment by an assistant as in M-VATS or grasping of tissue, and a quick operation using characteristic forceps and energy devices can be performed. The U-VATS operations in the present study were mostly performed by the same operator and operation team, and the operation time was further shortened as the number of cases increased. However, the multivariate logistic regression model showed that operation time and postoperative drainage duration were not significant predictors of postoperative pain.
This study has some limitations. First, this was a retrospective, non-randomized, single-institution study. Second, the study period was too short to evaluate long-term effectiveness. Evaluation of chronic pain and neuralgia is also needed.
In conclusion, U-VATS shortened operation time, postoperative drainage duration, and hospitalization compared with conventional M-VATS, and it significantly reduced the use of postoperative analgesics. There were no differences in perioperative results such as blood loss and the postoperative complication rate, and U-VATS can be said to be a safe and minimally invasive surgical procedure.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-2759
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-2759
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-20-2759
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-2759). HI serves as an unpaid editorial board member of Journal of Thoracic Disease from Aug 2020 to Jul 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Maebashi Red Cross Hospital (NO.: 2020-17) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rocco G, Martin-Ucar A, Passera E. Uniportal VATS wedge pulmonary resections. Ann Thorac Surg 2004;77:726-8. [Crossref] [PubMed]
- Gonzalez-Rivas D, Paradela M, Fieira E, et al. Single-incision video-assisted thoracoscopic lobectomy: initial results. J Thorac Cardiovasc Surg 2012;143:745-7. [Crossref] [PubMed]
- Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg. 2013;95:426-32. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fieira E, Delgado M, et al. Uniportal video-assisted thoracoscopic sleeve lobectomy and other complex resections. J Thorac Dis 2014;6:S674-81. [PubMed]
- Yang Y, Dong J, Huang Y. Single-incision versus conventional three-port video-assisted surgery in the treatment of pneumothorax: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg 2016;23:722-8. [Crossref] [PubMed]
- Nachira D, Ismail M, Meacci E, et al. Uniportal vs. triportal video-assisted thoracic surgery in the treatment of primary pneumothorax-a propensity matched bicentric study. J Thorac Dis 2018;10:S3712-9. [Crossref] [PubMed]
- Hirai K, Takeuchi S, Usuda J. Single-incision thoracoscopic surgery and conventional video-assisted thoracoscopic surgery: a retrospective comparative study of perioperative clinical outcomes†. Eur J Cardiothorac Surg 2016;49 Suppl 1:i37-i41. [PubMed]
- Zhao R, Shi Z, Cheng S. Uniport video assisted thoracoscopic surgery (U-VATS) exhibits increased feasibility, non-inferior tolerance, and equal efficiency compared with multiport VATS and open thoracotomy in the elderly non-small cell lung cancer patients at early stage. Medicine (Baltimore) 2019;98:e16137. [Crossref] [PubMed]
- Dai F, Meng S, Mei L, et al. Single-port video-assisted thoracic surgery in the treatment of non-small cell lung cancer: a propensity-matched comparative analysis. J Thorac Dis 2016;8:2872-8. [Crossref] [PubMed]
- Akter F, Routledge T, Toufektzian L, et al. In minor and major thoracic procedures is uniport superior to multiport video-assisted thoracoscopic surgery?. Interact Cardiovasc Thorac Surg 2015;20:550-5. [Crossref] [PubMed]
- Yang X, Li M, Yang X, et al. Uniport versus multiport video-assisted thoracoscopic surgery in the perioperative treatment of patients with T1-3N0M0 non-small cell lung cancer: a systematic review and meta-analysis. J Thorac Dis 2018;10:2186-95. [Crossref] [PubMed]
- Maguire MF, Ravenscroft A, Beggs D, et al. A questionnaire study investigating the prevalence of the neuropathic component of chronic pain after thoracic surgery. Eur J Cardiothorac Surg 2006;29:800-5. [Crossref] [PubMed]
- Sihoe AD, Cheung CS, Lai HK, et al. Incidence of chest wall paresthesia after needlescopic video-assisted thoracic surgery for palmar hyperhidrosis. Eur J Cardiothorac Surg 2005;27:313-9. [Crossref] [PubMed]
- Landreneau RJ, Mack MJ, Hazelrigg SR, et al. Prevalence of chronic pain after pulmonary resection by thoracotomy or video-assisted thoracic surgery. J Thorac Cardiovasc Surg 1994;107:1079-85; discussion 1085-6. [Crossref] [PubMed]


