Risk factors for acute kidney injury in overweight patients with acute type A aortic dissection: a retrospective study
Introduction
Acute kidney injury (AKI) after thoracic aortic surgery is a common complication and increases mortality (1-3). It develops in a variety of settings ranging from a minimal elevation in serum creatinine (sCr) to renal replacement therapy (RRT). The overall incidence of AKI after aortic surgery has been reported ranging from 18% to 55%, which is higher compared with other kinds of cardiac procedures (4,5). It affects patients’ prognosis as well as long-term mortality, even for patients with partial and complete recovery (2).
Beijing aortic disease center mainly deals with aortic diseases, especially aortic dissection. Many reports have shown the incidence and risk factors for AKI in patients with acute type A aortic dissection (TAAD), but always with different conclusions. All patients in our center with complicated TAAD need Sun’s procedure (6). The procedure refers to total aortic arch replacement using a tetra-furcated graft with implantation of a specially designed frozen elephant trunk in the descending aorta. Deep hypothermic circulatory arrest (DHCA) and selective cerebral perfusion (SCP) are the key factors in this procedure.
We have found that AKI is more likely to occur in overweight patients (BMI ≥24), however, there are few data on the incidence and risk factors of AKI in overweight patients with acute TAAD. This retrospective study is therefore designed to investigate the incidence and risk factors for AKI in overweight patients (BMI ≥24) who underwent Sun’s procedure.
Patients and methods
The Ethics Committees of Beijing Anzhen Hospital, Capital Medical University approved this retrospective study and waived the need for individual informed consent.
Patients
The medical records of patients (BMI ≥24) who underwent Sun’s procedure between December 2009 and April 2013 were reviewed retrospectively. Patients with a history of preoperative RRT were excluded, and 108 consecutive eligible patients comprised the cohort for the present analysis.
Diagnostic criteria for postoperative AKI
The postoperative AKI was diagnosed according to Acute Kidney Injury Network (AKIN), and the criteria and classification are shown in Table 1 in detail. The AKI criteria comprised an absolute increase in sCr of more than or equal to 0.3 mg/dL or a percentage increase in the sCr of more than or equal to 50%. We did not take urine output into consideration because of its inaccuracy.
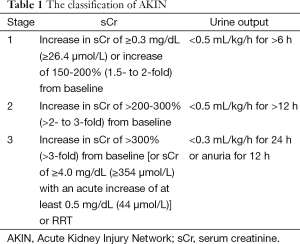
Full table
Sun’s procedure
All patients underwent Sun’s procedure. The procedure refers to total arch replacement using a tetra-furcate vascular graft in combination with implantation of a special stented graft into the descending aorta. Briefly, it is performed with right axillary artery cannulation for CPB and SCP under DHCA. This procedure involves implantation of the stented graft into the descending aorta, total arch replacement with a 4-branched vascular graft, a special sequence for aortic reconstruction (proximal descending aorta, then left carotid artery, ascending aorta, left subclavian artery, and finally innominate artery), and early rewarming and reperfusion after distal anastomosis to minimize cerebral and coronary ischemia. The time of SCP refers to the interval between the initiation of hypothermic circulatory arrest and completion of left carotid anastomosis, which is longer than the time of DHCA. In this period, lower body perfusion is arrested to implant the stented graft and suture the proximal descending anastomosis.
Statistical analysis
Values are expressed as mean ± standard deviation or the number of patients (%), as appropriate. Logistic regression models were used to identify univariate and multivariate predictors for AKI. Univariate logistic regression analysis was used first to identify possible risk factors for AKI, and the multivariate model included variables that were significant on univariate analysis. For all analyses, a probability value of less than 0.05 was considered statistically significant. SPSS 20.0 was used to analyze the data.
Results
One hundred and eight patients comprised the cohort for statistical analysis. The mean age was 43.69±9.66 years (19-75 years), 104 (96.3%) were male. Medical histories included hypertension (87.0%), DM (3.7%), Marfan syndrome (5.6%), cerebrovascular disease (0.9%), COPD (0.9%), coronary heart disease (6.5%), aortic rupture (0.9%), acute cardiac tamponade (26.9%), cerebral ischemia (5.6%), visceral ischemia (11.1%), lower extremity ischemia (6.5%), and previous cardiac operation (2.8%). Fifty-seven (52.8%) patients had aortic regurgitation (AI), 21 (19.4%) patients had mitral regurgitation (MI) and only 1 (0.9%) patient had tricuspid regurgitation (TI). No patient had peripheral vascular disease or spinal ischemia. Fifty-nine (54.6%) patients underwent emergency operations. All patients underwent Sun’s procedure, 34 (31.5%) combined with Bentall, 26 (24.1%) combined with aortic valvuloplasty (AVP) and 7 (6.5%) combined with other kinds of surgical procedures. The mean duration of CPB, aortic cross-clamping, and DHCA was respectively 202.94±43.89, 111.46±33.33, and 38.14±12.46 min. The mean value of sCr was 103.58±48.80 mmol/L before surgery, and 32 (29.6%) patients were found with renal injury. Seventy-two (66.7%) patients developed postoperative AKI and 15 (13.9%) patients required RRT.
The results of univariate analysis of risk factors for postoperative AKI are shown in Table 2. Elevated preoperative sCr level [odds ratio (OR), 1.310; 95% confidence interval (CI), 1.095-1.567; P=0.013] and 72-hour drainage volume (OR, 1.119; 95% CI, 1.020-1.227; P=0.029) were identified as the independent risk factors for postoperative AKI in the multivariate analysis (Table 3).
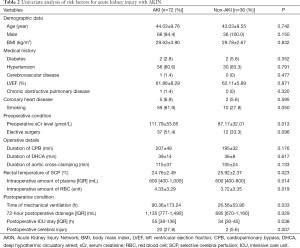
Full table
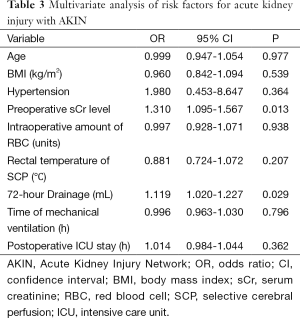
Full table
Comment
This retrospective study identified the incidence and risk factors for AKI in overweight patients with TAAD who underwent Sun’s procedure. Seventy-two (66.7%) patients developed postoperative AKI and 15 (13.9%) patients required RRT. The overall postoperative mortality rate was 7.4%, 8.3% in AKI group and 5.6% in non-AKI group. Multivariate logistic regression analysis demonstrated that elevated preoperative sCr level and 72-h drainage volume were independent risk factors for AKI.
Recent studies have reported the incidence and risk factors of postoperative AKI, however, no agreement has been reached yet. Nota and his colleagues (7) reported that the incidence of AKI in aortic arch surgery with SCP and mild hypothermic lower body circulatory arrest (HLBCA) was 43.1% and only two cases needed RRT, which are pretty lower than our results. Chronic kidney disease (CKD) and mild HLBCA time >60 min were identified as independent risk factors for postoperative AKI in that study. Another study reported an AKI incidence of 48% among 267 patients after aortic arch surgery with DHCA, including 36% of emergency surgeries and 36% of acute aortic dissections (8). However, Englberger (9) showed a much lower incidence of AKI (17.7%) and RRT (2.1%) in 851 patients undergoing elective thoracic aortic surgery with or without DHCA. Emergency surgery and TAAD were excluded out of their cohort as compared with our study, and it is therefore not surprising that a lower incidence of AKI (17.7%) was obtained.
The overall 30-day mortality was 7.4% (8 of 108 patients), which is similar to the study reported by Roh (10), but lower than that reported by another two previous studies of aortic surgery (11.1% and 13.5%, respectively) (11,12). Although several reports have documented the independent association between postoperative AKI and mortality in aortic surgery (11,13,14), no significant difference was observed in this study, probably because the number of patients might not be large enough. What is important is that postoperative AKI may increase the 10-year mortality risk (2) regardless of renal recovery at discharge. Obviously, identifying risk factors and preventing postoperative AKI are crucial to improve patients’ prognosis.
Elevated preoperative sCr level was identified as an independent risk factor for postoperative AKI in the logistic regression model, which is consistent with previous studies (11,15,16). Bove (15) reported that preoperative renal injury, defined as an sCr greater than 1.4 mg/dL, was associated with postoperative AKI, and Englberger (9) found that elevated preoperative sCr (>1.2 mg/dL) was associated with postoperative AKI univariately but not multivariately. In his study, independent risk factors for AKI included increased age, elevated BMI, hypertension, impaired LVEF, preoperative anemia and CPB duration, which were quite different from our study. We’d better pay more attention when dealing with such cases because we confirmed the relationship between elevated preoperative sCr level and postoperative AKI again in our study. Another independent risk factor was identified as 72-h drainage volume, which has not been reported previously. Decreasing the drainage volume after surgery may reduce the incidence of postoperative AKI.
Several variables associated with AKI in the univariate analysis were not significant in the multivariate analysis. Postoperative ICU stay was not an independent risk factor although a more than 2-fold increased risk was observed in the univariate analysis. In our study, DHCA was not associated with AKI, which is consistent with the study reported by Englberger (9). However, Mori (17) identified DHCA as an independent risk factor for postoperative AKI. DHCA prolongs the duration of CPB and may reduce the activity of enzymes involved in platelet activation pathways and clotting factors, increasing transfusion requirements (18), but we did not find this in our study. Tsai (19) reported that moderate hypothermia with SCP was associated with lower in-hospital and 30-day mortality, shorter duration of CPB, and fewer neurologic complications than deep hypothermia in patients who underwent aortic arch surgery with ACP.
Fifteen (13.9%) patients in this study required postoperative RRT. The incidence was higher than that in other studies, ranging from 2% to 8% (9,11,20). Roh (10) suggested that prompt application of RRT might improve outcomes, however, there are still no definite standards on when to initiate RRT and large clinical studies are needed to confirm the effect of early intervention of RRT.
This study has its limitations. Firstly, our center takes Sun’s procedure as the standard procedure for complicated TAAD while other centers choose other different surgical procedures, and this may lead to differences with other studies. Secondly, the number [108] of patients may be a little small for this retrospective study. However, it did have a homogeneous population undergoing Sun’s procedure for acute TAAD with SCP and DHCA, and the results are trustworthy. Thirdly, many other studies (4,10,21) took RIFLE classification as the diagnostic criteria for AKI while in this study we chose AKIN, which might be another factor affecting the results. The last aspect was that our study was mainly focused on overweight patients (BMI ≥24). We always found that the overweight patients were more likely to develop postoperative AKI in clinical work and elevated BMI was identified as one of the independent risk factors for postoperative AKI (9). The high incidence of postoperative AKI (66.7%) in our study confirms that overweight patients are more prone to develop AKI. This may be the key factor resulting in the higher incidence of postoperative AKI.
Early diagnosis of AKI may contribute to timely intervention and improve the prognosis (20). The prevalent diagnosis of postoperative AKI is based on sCr level, glomerular filtration rate (GFR) and urine output, with the absence of accurate tests that can be performed early in the process of AKI and predict outcome. Recently several biomarkers have been studied as possible tools for the early diagnosis of AKI. In those biomarkers, promising results have been reported for neutrophil gelatinase-associated lipocalin (NGAL) and cystatin C (22-25). Urine NGAL appears to be valuable in the prediction of occurrence, duration and severity of AKI while serum cystatin C predicts AKI 1 to 2 days earlier than sCr. All those studies were performed in cardiac procedures except for TAAD and few studies have been performed to confirm the diagnostic value of these biomarkers in TAA D.
Until now, this is the first time to report the incidence and risk factors for AKI after Sun’s procedure for TAAD with SCP and DHCA in overweight patients. The incidence (66.7%) in this study was higher than that ever reported, and it reminds us of paying more attention to prevent postoperative AKI in overweight patients. Elevated preoperative sCr level is an independent risk factor for postoperative AKI, and decreasing 72-h drainage volume may reduce the incidence of postoperative AKI as well. Furthermore, this study is just a beginning, and the most important is the early diagnosis and prevention.
Acknowledgements
Funding: This work was supported by grants from project of International Science and Technology Cooperation Program of China (2012DFA31110) and the research special fund for public welfare industry of health (201402009).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: L Sun, Y Liu, J Zhu.
References
- Chertow GM, Levy EM, Hammermeister KE, et al. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med 1998;104:343-8. [PubMed]
- Hobson CE, Yavas S, Segal MS, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation 2009;119:2444-53. [PubMed]
- Mariscalco G, Lorusso R, Dominici C, et al. Acute kidney injury: a relevant complication after cardiac surgery. Ann Thorac Surg 2011;92:1539-47. [PubMed]
- Kuitunen A, Vento A, Suojaranta-Ylinen R, et al. Acute renal failure after cardiac surgery: evaluation of the RIFLE classification. Ann Thorac Surg 2006;81:542-6. [PubMed]
- Kim MY, Jang HR, Huh W, et al. Incidence, risk factors, and prediction of acute kidney injury after off-pump coronary artery bypass grafting. Ren Fail 2011;33:316-22. [PubMed]
- Ma WG, Zheng J, Dong SB, et al. Sun’s procedure of total arch replacement using a tetrafurcated graft with stented elephant trunk implantation: analysis of early outcome in 398 patients with acute type A aortic dissection. Ann Cardiothorac Surg 2013;2:621-8. [PubMed]
- Nota H, Asai T, Suzuki T, et al. Risk factors for acute kidney injury in aortic arch surgery with selective cerebral perfusion and mild hypothermic lower body circulatory arrest. Interact Cardiovasc Thorac Surg 2014;19:955-61. [PubMed]
- Kowalik MM, Lango R, Klajbor K, et al. Incidence- and mortality-related risk factors of acute kidney injury requiring hemofiltration treatment in patients undergoing cardiac surgery: a single-center 6-year experience. J Cardiothorac Vasc Anesth 2011;25:619-24. [PubMed]
- Englberger L, Suri RM, Greason KL, et al. Deep hypothermic circulatory arrest is not a risk factor for acute kidney injury in thoracic aortic surgery. J Thorac Cardiovasc Surg 2011;141:552-8. [PubMed]
- Roh GU, Lee JW, Nam SB, et al. Incidence and risk factors of acute kidney injury after thoracic aortic surgery for acute dissection. Ann Thorac Surg 2012;94:766-71. [PubMed]
- Arnaoutakis GJ, Bihorac A, Martin TD, et al. RIFLE criteria for acute kidney injury in aortic arch surgery. J Thorac Cardiovasc Surg 2007;134:1554-60; discussion 1560-1. [PubMed]
- Augoustides JG, Pochettino A, Ochroch EA, et al. Renal dysfunction after thoracic aortic surgery requiring deep hypothermic circulatory arrest: definition, incidence, and clinical predictors. J Cardiothorac Vasc Anesth 2006;20:673-7. [PubMed]
- Black SA, Brooks MJ, Naidoo MN, et al. Assessing the impact of renal impairment on outcome after arterial intervention: a prospective review of 1,559 patients. Eur J Vasc Endovasc Surg 2006;32:300-4. [PubMed]
- Augoustides JG, Pochettino A, Ochroch EA, et al. Clinical predictors for prolonged intensive care unit stay in adults undergoing thoracic aortic surgery requiring deep hypothermic circulatory arrest. J Cardiothorac Vasc Anesth 2006;20:8-13. [PubMed]
- Bove T, Calabrò MG, Landoni G, et al. The incidence and risk of acute renal failure after cardiac surgery. J Cardiothorac Vasc Anesth 2004;18:442-5. [PubMed]
- Elhmidi Y, Bleiziffer S, Piazza N, et al. Incidence and predictors of acute kidney injury in patients undergoing transcatheter aortic valve implantation. Am Heart J 2011;161:735-9. [PubMed]
- Mori Y, Sato N, Kobayashi Y, et al. Acute kidney injury during aortic arch surgery under deep hypothermic circulatory arrest. J Anesth 2011;25:799-804. [PubMed]
- Kamiya H, Hagl C, Kropivnitskaya I, et al. The safety of moderate hypothermic lower body circulatory arrest with selective cerebral perfusion: a propensity score analysis. J Thorac Cardiovasc Surg 2007;133:501-9. [PubMed]
- Tsai JY, Pan W, Lemaire SA, et al. Moderate hypothermia during aortic arch surgery is associated with reduced risk of early mortality. J Thorac Cardiovasc Surg 2013;146:662-7. [PubMed]
- Bennett M, Dent CL, Ma Q, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol 2008;3:665-73. [PubMed]
- D’Onofrio A, Cruz D, Bolgan I, et al. RIFLE criteria for cardiac surgery-associated acute kidney injury: risk factors and outcomes. Congest Heart Fail 2010;16:S32-6. [PubMed]
- Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005;365:1231-8. [PubMed]
- Wagener G, Jan M, Kim M, et al. Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology 2006;105:485-91. [PubMed]
- Dent CL, Ma Q, Dastrala S, et al. Plasma neutrophil gelatinase-associated lipocalin predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: a prospective uncontrolled cohort study. Crit Care 2007;11:R127. [PubMed]
- Obermüller N, Geiger H, Weipert C, et al. Current developments in early diagnosis of acute kidney injury. Int Urol Nephrol 2014;46:1-7. [PubMed]


