
The value of cardiopulmonary exercise testing in the diagnosis of pulmonary hypertension
Introduction
Pulmonary hypertension (PH) refers to a group of pathophysiological syndromes of multiple etiologies. It is characterized by pulmonary vascular remodeling, which causes pulmonary vascular resistance to progressively increase, leading to right heart failure and death (1). Patients with PH usually complain of dyspnea on exertion-a none specific symptom that easily leads to missed diagnosis and misdiagnosis. At diagnosis, 85% of high-risk PH patients are already at an advanced stage of the disease (2,3). Therefore, the screening and timely identification of suspected PH patients are imperative. Right heart catheterization (RHC) is the gold standard for the diagnosis of PH, but it can also cause complications.
Echocardiography is the most commonly used screening method for PH in the clinical settings. However, it often leads to the overestimation and underestimation of pulmonary artery systolic pressure (4). Held et al. (5) retrospectively analyzed the echocardiographic data of 42 patients with chronic thromboembolic pulmonary hypertension (CTEPH) and showed that echocardiography detected CTEPH in 29 patients (69%), while the other 13 patients (31%) went undiagnosed. The European Society of Cardiology proposed that echocardiography was not suitable for screening patients with mild asymptomatic PH (6). Therefore, more accurate screening methods need to be found. CPET may improve diagnostic specificity in patients with echocardiography-suspected PH.
In recent years, several studies have confirmed the important role of cardiopulmonary exercise testing (CPET) in PH diagnosis. Woods et al. (7) compared the CPET data of 40 PH patients and 25 healthy controls, and found significantly lower end-tidal carbon dioxide partial pressure (PetCO2) and higher minute ventilation (VE)/carbon dioxide output (VCO2) in the PH patients. Meanwhile, the levels of PetCO2 and VE/VCO2 were found to be correlated with the severity of PH. Nishio et al. (8) demonstrated that PH patients had a decreased peak oxygen uptake (VO2) and an increased VE/VCO2 slope compared with chronic heart failure patients. CPET variables were also shown to be associated with hemodynamic parameters. Thirapatarapong et al. (9) reviewed and analyzed the data on pulmonary function, RHC, and CPET in 98 patients with severe chronic obstructive pulmonary disease. They observed that chronic obstructive pulmonary disease patients with PH had a significantly reduced peak work rate (WR), peak VO2, and peak oxygen pulse (O2 pulse). Moreover, peak VO2 was negatively correlated with mean pulmonary artery pressure (mPAP). Accordingly, CPET is expected to serve as a noninvasive but effective means of identifying pulmonary vasculopathy in PH. In this study, we aimed to explore the value of CPET in the diagnosis of PH.
We present the following article in accordance with the STARD reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-1061b).
Methods
This single center study was conducted at Fuwai Hospital, National Center for Cardiovascular Diseases in Beijing, China. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Committee Board of the Fuwai Hospital. Informed consent was given by all patients.
Study sample
Untreated patients with suspected PH who were admitted to Fuwai Hospital between January 2017 and August 2018 were consecutively included in this study. Patients with any of the following conditions were considered to be suspected PH cases: (I) exertional dyspnea as the chief complaint; (II) P2 enhancement and pathological third heart sound during physical examination; (III) elevated levels of plasma B-type natriuretic peptide or N-terminal prohormone of brain natriuretic peptide (NT-proBNP); (IV) electrocardiogram manifestations, such as right axis deviation, right bundle branch block, and other phenomena, reflecting an increased right heart load; (V) protruding pulmonary artery segment and expanded right heart image from chest X-ray; (VI) reduced pulmonary diffusion capacity; (VII) suspected PH by echocardiography, including tricuspid regurgitation velocity (TRV) >2.8 m/s, a widened pulmonary artery, a dilated right heart, and a widened inferior vena cava, etc.; (VIII) patients at high-risk of PH.
All patients were over the age of 18. Patients with any of the following conditions were excluded: (I) recurrent syncope or massive hemoptysis; (II) neuromuscular disease affecting the 6-minute walk test and CPET; (III) severe arrhythmia requiring intervention; (IV) severe liver and kidney dysfunction; (V) severe anemia (hemoglobin <90 g/L). Patients who had recently received exercise rehabilitation training were also excluded.
Echocardiography and RHC
Patients’ echocardiographic and hemodynamic parameters were collected in addition to their age, sex, body mass index (BMI), 6-minute walking distance (6-MWD), World Health Organization functional class (WHO FC), and plasma levels of NT-proBNP. As a screening tool for PH, echocardiography was performed on each patient on the day of admission. Patients who had a TRV >2.8 m/s and other echocardiographic signs such as a widened pulmonary artery, dilated right heart, and widened inferior vena cava were considered to have PH (10). The diastolic left ventricle diameter was measured in the left ventricular long-axis view, and the diastolic right ventricle diameter was measured in the apical four-chamber view. Ejection fraction was assessed using the Simpson biplane method.
The diagnosis of PH in each patient was confirmed by RHC. As the gold standard for PH diagnosis, RHC was conducted by experienced pulmonary vascular physicians. The hemodynamic parameters obtained by RHC included right atrial pressure, mPAP, total pulmonary resistance, cardiac index, and mixed venous oxygen saturation. As assessed by RHC, mPAP ≥25 mmHg at rest was defined as PH (11). Physicians who conducted RHC were blind to the CPET information of the participants.
CPET
Before RHC, each suspected PH patient enrolled in this study underwent symptom-limited CPET using the COSMED Quark CPET system. The performers of CPET were blind to the RHC information of the enrolled patients. The following four phases were completed on a cycle ergometer: (I) 3 minutes of rest; (II) 3 minutes of unloaded pedaling at an approximate speed of 60 rpm; (III) WR-incremental exercise to achieve maximal tolerance; (IV) 5 minutes of recovery. VE, VO2, and VCO2 were measured breath-by-breath and were averaged every 10 seconds during the entire process. Meanwhile, the responses of the cardiovascular system including blood pressure, heart rate (HR), and a 12-lead electrocardiogram were recorded.
Peak VO2 was defined as the highest 30-second average value of VO2 during the final minute of exercise. The anaerobic threshold (AT) was the maximal VO2 before the onset of lactic acidosis, which was determined using the V-slope method. The peak O2 pulse was the ratio of peak VO2 to peak HR. The VE/VCO2 slope was the linear regression slope of the relation of VE to VCO2 over the whole exercise period. The oxygen uptake efficiency slope (OUES) was the slope in the following equation: VO2 = OUES × VE + B. The peak respiratory exchange ratio (RER) was defined as the ratio of peak VCO2 to peak VO2. Heart rate recovery (HRR) was considered as the maximum HR minus the HR at 2 minutes after peak exercise. Peak circulatory power was defined as the product of peak VO2 and peak systolic blood pressure (SBP). Peak ventilatory power was defined as peak SBP divided by the VE/VCO2 slope (12). Ventilation efficiency was assessed according to the VE/VCO2 ratio, VE/VCO2 slope, and PetCO2.
Statistical analysis
Continuous variables are presented as the mean ± standard deviation or median (interquartile range), and categorical variables are presented as counts or percentages. To compare the differences between groups, an independent sample t-test was used for continuous variables with normal distribution, a non-parametric Kruskal-Wallis test was used for continuous variables with non-normal distribution, and the chi-square test was used for categorical variables. Univariate and multivariate logistic regression analyses were carried out to identify the CPET variables that were independently associated with PH. Furthermore, linear correlation analysis between CPET variables and mPAP was performed. Receiver operating characteristic (ROC) curve analysis was used to determine the cutoff values, sensitivity, and specificity of CPET variables for diagnosing PH. Missing data are processed using weights. P<0.05 was defined as statistically significant. All statistical analyses were carried out with SPSS 22.0 software (SPSS, Inc., Chicago, IL, USA).
Results
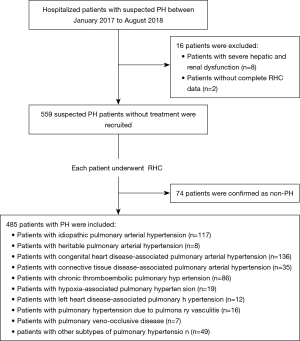
In total, 559 suspected PH patients without treatment were recruited. Of them, 485 patients were confirmed as PH by RHC, including 136 patients with congenital heart disease-associated pulmonary arterial hypertension, 117 with idiopathic pulmonary arterial hypertension, 86 with CTEPH, 35 with connective tissue disease-associated pulmonary arterial hypertension, 19 with hypoxia-associated PH, 16 with PH due to pulmonary vasculitis, 12 with left heart disease-associated PH, 8 with heritable pulmonary arterial hypertension, 7 with pulmonary veno-occlusive disease, and 49 with other subtypes of PH. The participant enrollment and exclusion processes are detailed in Figure 1.
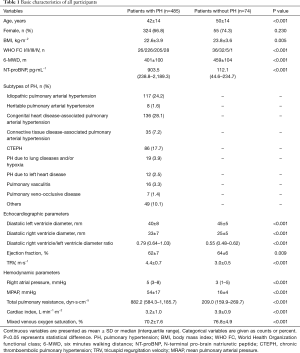
Baseline characteristics of patients
Baseline characteristics were compared between patients with and without PH. PH patients were younger (42±14 vs. 50±14 years, P<0.001) and had a lower BMI (22.6±3.9 vs. 23.8±3.6 kg·m−2, P=0.005), a shorter 6-MWD (401±100 vs. 459±104 m, P<0.001), a higher NT-proBNP [903.5 (238.8–2189.3) vs. 112.1 (44.6–234.7), P<0.001], and a poorer WHO FC (P<0.001). The echocardiographic and hemodynamic parameters of the two groups were also statistically significantly different. The details are displayed in Table 1.
Full table
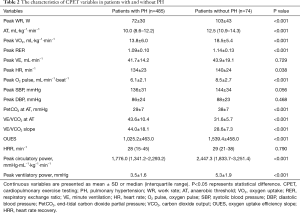
CPET characteristics of patients with PH
The time interval between CPET and RHC was less than 7 days. No adverse events resulted from performing both of them. Table 2 compares the CPET variables between patients with and without PH. Besides peak VE, peak SBP, peak diastolic blood pressure (DBP), and HRR, the other CPET variables were statistically different. Patients with PH had decreased exercise tolerance, which was mainly reflected by a significant decrease in peak WR (72±30 vs. 103±43 W, P<0.001), AT [10.0 (8.6–12.2) vs. 12.5 (10.9–14.3) mL·kg−1·min−1, P<0.001], peak VO2 (13.8±6.0 vs. 18.5±5.4 mL·kg−1·min−1, P<0.001), and OUES (1,025.2±463.0 vs. 1,539.4±458.0, P<0.001). PH patients also showed poor circulatory response, which was supported by significant decreases in peak HR (134±23 vs. 140±24 min−1, P=0.038), O2 pulse (6.1±2.1 vs. 8.5±2.7 mL·min−1·beat−1, P<0.001), and peak circulatory power [1,776.0 (1,341.2–2,293.2) vs. 2,447.3 (1,833.7–3,251.4) mmHg·mL−1·kg−1·min−1, P<0.001]. In addition, ineffective ventilation was increased in PH patients, which was reflected by significant decreases in PetCO2 at AT (29±7 vs. 38±7mmHg, P<0.001) and peak ventilatory power (3.5±1.6 vs. 5.3±1.9 mmHg, P<0.001), along with significant increases in VE/VCO2 at AT (43.6±10.4 vs. 31.6±5.7, P<0.001) and the VE/VCO2 slope (44.0±18.1 vs. 28.6±7.3, P<0.001).
Full table
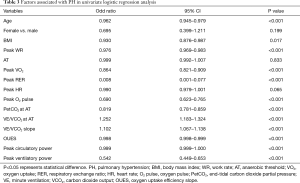
CPET variables independently associated with PH
As shown in Table 3, univariate logistic regression analysis revealed that age, BMI, and CPET variables except for AT and peak HR were associated with PH (all P<0.001). Age, sex, BMI, peak WR, peak VO2, peak RER, O2 pulse, PetCO2 at AT, the VE/VCO2 at AT, VE/VCO2 slope, peak circulatory power and peak ventilatory power were subsequently included in multivariate logistic regression analysis. After adjustment for age, sex and BMI, CPET variables including peak WR, peak VO2, and PetCO2 at AT were independently associated with PH (Table 4). In addition, the above three CPET variables were significantly correlated with the mPAP measured by RHC, the lower the peak WR, peak VO2 and PetCO2 at AT, the higher the mPAP value (Figure 2).
Full table

Full table

ROC curve analysis of CPET variables in PH diagnosis
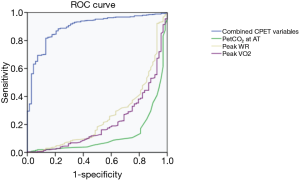
The ROC curve analysis of CPET variables in the diagnosis of PH was performed using the gold standard mentioned previously. The sensitivity, specificity, and the area under the receiver operator characteristic curve (AUC) of CPET variables in PH diagnosis are shown in Table 5. A regression equation was obtained from multivariate ROC curve analysis as follows: Y=9.294 − 0.0048 × peak WR − 0.0173 × peak VO2 − 0.0752 × PetCO2 at AT. Combining with the above three CPET variables, this model had the highest AUC [0.890 (0.852–0.927), P<0.001] and high sensitivity (81.8%) and specificity (86.5%) for diagnosing PH, as shown in Figure 3.

Full table
Subgroup analyses of missed diagnosis and misdiagnosis of PH by echocardiography
Patients with TRV≤2.8 m·s−1 were not considered to have PH. However, among 59 patients whose TRV≤2.8 m·s−1, 28 patients were confirmed to have PH by RHC. The cutoff values of CPET variables could identify a subset of patients with normal echocardiography (Table 6). Patients with TRV >2.8 m·s−1 together with other echocardiographic signs were considered to have PH. Forty-three patients screened as PH by echocardiography were confirmed to have a normal mPAP by RHC. The cutoff values of CPET variables could also identify a subset of patients misdiagnosed as PH by echocardiography (Table 7).

Full table

Full table
Discussion
PH presents similar lesions like wall thickening and luminal narrowing of the pulmonary arterioles are presented regardless of the specific pathogenetic mechanism (13). This pulmonary vascular remodeling leads to an increase in pulmonary vascular resistance and a corresponding increase in pulmonary artery pressure. Right heart function becomes impaired by the increased right ventricular afterload, and an enlarged right ventricle causes the interventricular septum to shift leftward, which affects left heart filling. Both conditions affect the patient's cardiac output, thus leading to reduced VO2 (14). In addition, pulmonary vascular lesions cause a pulmonary ventilation/ perfusion mismatch and enlargement of a physiological dead space, thus reducing ventilation efficiency (15). Recent studies have found consistent changes in many CPET variables in patients with PH (16-18). In our study, PH patients presented with significantly reduced peak WR, AT, peak VO2, O2 pulse, and PetCO2 at AT, and significantly increased VE/VCO2 at AT and VE/VCO2 slope, compared to non-PH patients. These findings are consistent with the results of previous studies. The value of novel CPET variables including OUES, peak circulatory power and peak ventilatory power were also investigated in this study. Previous research shows that the OUES is significantly lower in patients with heart failure and is also related to the severity of heart failure (19). Borghi-Silva et al. (20) analyzed the CPET and echocardiographic data of 86 heart failure patients with reduced ejection fraction. They found a peak ventilatory power to be significantly decreased, reflecting the impaired right heart function and pulmonary hemodynamic deteriorations in these patients. Cohen-Solal et al. (21) proposed that, of the many CPET variables, peak circulatory power is the best indicator for predicting adverse clinical outcomes in PH. In this study, we found that the three novel CPET variables were significantly reduced in PH patients, which supports the previous findings.
In a 2010 review published by Arena et al. (22), 19 studies described the reliability of CPET in identifying pulmonary vasculopathy, and its value in diagnosing different PH subtypes. Several studies have demonstrated a significant correlation between CPET variables and hemodynamic parameters. Yasunobu et al. (23) found that in PH patients, PetCO2 at rest, at AT and at peak exercise was negatively correlated with mPAP (all P<0.05). An article published by Holverda et al. (24) indicated that the lowest VE/VCO2 was significantly correlated with mPAP (r=0.43, P<0.05). Gläser et al. (25) investigated patients with pulmonary fibrosis and found that the VE/VCO2 slope (r=0.77, P<0.05) and peak VO2 (r=−0.52, P<0.05) was significantly correlated with pulmonary artery systolic pressure in patients who had pulmonary arterial hypertension due to pulmonary fibrosis. As above, our study also found that peak WR, peak VO2, peak RER, peak O2 pulse, PetCO2 at AT, OUES, peak circulatory power, and peak ventilatory power were negatively correlated with mPAP in all included patients with suspected PH, while VE/VCO2 at AT and the VE/VCO2 slope were positively correlated with mPAP (data not shown). Of these, peak WR, peak VO2 and PetCO2 at AT were founded to be independently associated with PH. A study conducted by Zhao et al. (26) included 88 patients with echocardiography-suspected PH. The results showed that the combination of the VE/VCO2 slope and AT achieved a sensitivity and specificity of 93% and 95%, respectively, for diagnosing PH with RHC used as the gold standard. In our study, the combination of the CPET variables including peak WR, peak VO2, and PetCO2 at AT also had high sensitivity and specificity in PH diagnosis.
Sciumè et al. (27) conducted a follow-up study in patients with primary myelofibrosis and found that patients with normal baseline echocardiography but abnormal CPET developed PH after 12 months of follow-up. Their results demonstrated that CPET was more sensitive and specific than echocardiography in the identification of pulmonary vascular lesions. In the present study, the regression equation Y=9.294 − 0.0048 × peak WR − 0.0173 × peak VO2 − 0.0752 × PetCO2 at AT achieved the highest AUC, which showed improved sensitivity and specificity in diagnosing PH. When Y was >0.86, it supported the PH diagnosis. We conducted subgroup analyses of patients who had a missed diagnosis or misdiagnosis PH by echocardiography. The cutoff value (Y>0.86) of the combined CPET variables including peak WR, peak VO2, and PetCO2 identified 19 out of 28 patients who had a normal echocardiography but who were confirmed as PH by RHC. However, the cutoff value (Y≤0.86) identified 36 out of 49 non-PH patients who were misdiagnosed as PH by echocardiography. Therefore, the negative predictive value of the combined CPET variables for PH diagnosis is higher. This shows that CPET is able to identify patients who are misdiagnosed as PH by echocardiography, and these patients can therefore be spared from invasive RHC, thus reducing the psychological and financial burden on patients and their families.
Limitations
One significant limitation of the study is the use of sample with suspected PH. Thus, the identified CPET parameters may be more valuable for the identification of PH from suspected PH. Patients with PH in our study had many subtypes due to different etiologies, the degree of VO2 decline and the severity of pulmonary ventilation/ perfusion mismatch might vary. But the PH patients’ number in different subtypes had a big difference. Thus, subgroup analyses were not conducted. In future prospective study, the diagnostic value of CPET in each subtype of PH will be explored respectively. In addition, our study investigated only the commonly used clinical CPET variables. The other significant limitation is no external validation sample for testing the external validity of CPET. The accuracy of CPET cutoff values for PH diagnosis needs to be validated in multiple centers.
The authors may consider subgroup analyses according to subtypes of PH. The diagnostic value for PH subtypes is also interesting.
Conclusions
CPET has significant value as a non-invasive method for the diagnosis of PH. Together with echocardiography, it can reduce the rates of missed diagnosis and misdiagnosis in patients with suspected PH, thus helping clinicians to accurately predict diagnosis and formulate appropriate treatment plans.
Acknowledgments
Funding: This work was supported by Beijing Municipal Science and Technology Project [grant number Z181100001718200].
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1061b
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1061b
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1061b). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by committee board of the Fuwai Hospital (NO.: 2018-BKJ09) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation 2010;121:2045-66. [Crossref] [PubMed]
- Deaño RC, Glassner-Kolmin C, Rubenfire M, et al. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral study. JAMA Intern Med 2013;173:887-93. [Crossref] [PubMed]
- Maron BA, Choudhary G, Khan UA, et al. Clinical profile and underdiagnosis of pulmonary hypertension in US veteran patients. Circ Heart Fail 2013;6:906-12. [Crossref] [PubMed]
- Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med 2009;179:615-21. [Crossref] [PubMed]
- Held M, Grun M, Holl R, et al. Cardiopulmonary exercise testing to detect chronic thromboembolic pulmonary hypertension in patients with normal echocardiography. Respiration 2014;87:379-87. [Crossref] [PubMed]
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension. Rev Esp Cardiol (Engl Ed) 2016;69:177. [Crossref] [PubMed]
- Woods PR, Frantz RP, Taylor BJ, et al. The usefulness of submaximal exercise gas exchange to define pulmonary arterial hypertension. J Heart Lung Transplant 2011;30:1133-42. [Crossref] [PubMed]
- Nishio R, Tanaka H, Tsuboi Y, et al. Differences in hemodynamic parameters and exercise capacity between patients with pulmonary arterial hypertension and chronic heart failure. J Cardiopulm Rehabil Prev 2012;32:379-85. [Crossref] [PubMed]
- Thirapatarapong W, Armstrong HF, Bartels MN. Comparing cardiopulmonary exercise testing in severe COPD patients with and without pulmonary hypertension. Heart Lung Circ 2014;23:833-40. [Crossref] [PubMed]
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685-713. [Crossref] [PubMed]
- Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013;62:D42-50. [Crossref] [PubMed]
- Guazzi M, Arena R, Halle M, et al. 2016 focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur Heart J 2018;39:1144-61. [Crossref] [PubMed]
- Vonk Noordegraaf A, Groeneveldt JA, Bogaard HJ. Pulmonary hypertension. Eur Respir Rev 2016;25:4-11. [Crossref] [PubMed]
- Chatterjee NA, Murphy RM, Malhotra R, et al. Prolonged mean VO2 response time in systolic heart failure: an indicator of impaired right ventricular-pulmonary vascular function. Circ Heart Fail 2013;6:499-507. [Crossref] [PubMed]
- Guazzi M, Bandera F, Ozemek C, et al. Cardiopulmonary Exercise Testing: What Is its Value? J Am Coll Cardiol 2017;70:1618-36. [Crossref] [PubMed]
- Farina S, Correale M, Bruno N, et al. The role of cardiopulmonary exercise tests in pulmonary arterial hypertension. Eur Respir Rev 2018;27:170134. [Crossref] [PubMed]
- Paolillo S, Farina S, Bussotti M, et al. Exercise testing in the clinical management of patients affected by pulmonary arterial hypertension. Eur J Prev Cardiol 2012;19:960-71. [Crossref] [PubMed]
- Weatherald J, Farina S, Bruno N, et al. Cardiopulmonary Exercise Testing in Pulmonary Hypertension. Ann Am Thorac Soc 2017;14:S84-92. [Crossref] [PubMed]
- Van Laethem C, Bartunek J, Goethals M, et al. Oxygen uptake efficiency slope, a new submaximal parameter in evaluating exercise capacity in chronic heart failure patients. Am Heart J 2005;149:175-80. [Crossref] [PubMed]
- Borghi-Silva A, Labate V, Arena R, et al. Exercise ventilatory power in heart failure patients: functional phenotypes definition by combining cardiopulmonary exercise testing with stress echocardiography. Int J Cardiol 2014;176:1348-9. [Crossref] [PubMed]
- Cohen-Solal A, Tabet JY, Logeart D, et al. A non-invasively determined surrogate of cardiac power ('circulatory power') at peak exercise is a powerful prognostic factor in chronic heart failure. Eur Heart J 2002;23:806-14. [Crossref] [PubMed]
- Arena R, Lavie CJ, Milani RV, et al. Cardiopulmonary exercise testing in patients with pulmonary arterial hypertension: an evidence-based review. J Heart Lung Transplant 2010;29:159-73. [Crossref] [PubMed]
- Yasunobu Y, Oudiz RJ, Sun XG, et al. End-tidal PCO2 abnormality and exercise limitation in patients with primary pulmonary hypertension. Chest 2005;127:1637-46. [Crossref] [PubMed]
- Holverda S, Bogaard HJ, Groepenhoff H, et al. Cardiopulmonary exercise test characteristics in patients with chronic obstructive pulmonary disease and associated pulmonary hypertension. Respiration 2008;76:160-7. [Crossref] [PubMed]
- Gläser S, Noga O, Koch B, et al. Impact of pulmonary hypertension on gas exchange and exercise capacity in patients with pulmonary fibrosis. Respir Med 2009;103:317-24. [Crossref] [PubMed]
- Zhao QH, Wang L, Pudasaini B, et al. Cardiopulmonary exercise testing improves diagnostic specificity in patients with echocardiography-suspected pulmonary hypertension. Clin Cardiol 2017;40:95-101. [Crossref] [PubMed]
- Sciumè M, Mattiello V, Cattaneo D, et al. Early detection of pulmonary hypertension in primary myelofibrosis: The role of echocardiography, cardiopulmonary exercise testing, and biomarkers. Am J Hematol 2017;92:E47-8. [Crossref] [PubMed]


