
Preoperative evaluation and indications for pulmonary metastasectomy
Introduction
Ever since Barney and Churchill described the first successful pulmonary metastasectomy (PM) case in 1939, PM has become established as a viable treatment that provides improved long-term survival (1,2). However, there has been no evidence based on randomized trials to indicate that PM is indeed the best treatment for patients with pulmonary metastases. Although many observational studies have presented favorable results with PM, these studies were limited by bias, such as patient selection bias (3).
Over the past two decades, remarkable advancements in drug therapy for various cancers have been made (4). In terms of local control of pulmonary metastases, stereotactic body radiation therapy (SBRT) has recently become widely performed as it was found to provide a favorable local control rate (5).
With this, thoracic surgeons are recommended to review their approach to the preoperative evaluation and the indications.
Preoperative evaluation
History and physical examinations
The basics of preoperative evaluation include taking a patient’s medical history and performing a physical examination. Detailed information on the treatment history for primary tumors, including the treatment mode, tumor stage and histologic type, time interval between the treatment for primary tumor and the detection of pulmonary metastases, presence of other metastatic sites, and the regimen and timing of chemotherapy, is necessary, as these can indicate important prognostic factors (6). Determining any past or present comorbidities, such as cerebrovascular or cardiovascular diseases, pulmonary diseases, diabetes mellitus, and renal or liver diseases, is also essential for assessing patients’ ability to tolerate surgery. Assessing the smoking history is important, as it correlates with various comorbidities. Furthermore, a current smoking history predisposes the patient to a high risk of developing postoperative complications (7). Medications currently being taken should be determined, and a perioperative plan concerning antiplatelets, anticoagulants, and immunosuppressants should be made. The recent administration of systemic treatment should also be precisely evaluated.
After cytotoxic chemotherapy, pulmonary resection should be planned following the recovery from a drop in the white blood cell count (generally about four weeks). In cases receiving bevacizumab, pulmonary resection should be planned at least six weeks after the last administration in order to reduce the risk of postoperative pulmonary fistula (8).
Although most candidates for PM do not present with any symptoms caused by pulmonary metastatic lesions, the presence of respiratory symptoms indicative of endobronchial involvement or centrally located bulky lesions should be investigated. Information on the activities of daily life should also be obtained from both the patient and the patients’ family. If the patient is capable of only limited self-care or is confined to a bed or chair for more than 50% of waking hours (ECOG Performance Status 3), the patient is not indicated for surgery in general.
Physiological tests
Generally, the physiological indications can be determined in line with those for pulmonary resection of lung cancer (9,10). Objective and convenient assessments for cardiopulmonary function, such as the 6-minute walk test or stair-climbing test, are useful for determining the physiological indications for pulmonary resection (11). A patient’s ability to tolerate pulmonary resection is decided based on cardiovascular evaluation and spirometry, in order to measure the diffusing capacity of carbon monoxide (DLco) and the forced expiratory volume in 1 second (FEV1). Because candidates for PM often receive chemotherapy as treatment for the primary tumor, damage due to chemotherapy, such as cardiac toxicity from anthracycline, should be properly assessed. Smoking-related cancers, such as esophageal cancer and head and neck cancer, are associated with higher incidences of comorbidities such as hypertension and chronic obstructive pulmonary disease than non-smoking-related cancers, so the cardiopulmonary function should be carefully assessed in these patients (12-14).
The evaluation of pulmonary nodules in patients with a history of malignancy
Differential diagnoses are needed when pulmonary nodules are detected in patients with a history of malignancy. If there are multiple nodules, metastatic disease from the primary tumor is a possible diagnosis, but when there is only one, it becomes difficult to distinguish pulmonary metastases from primary lung cancer. Upon detection of a solitary pulmonary nodule (SPN), whether or not a further invasive examination procedure should be done must be based on the subsequent treatment strategy for the SPN. That treatment strategy is decided after considering various factors, such as the primary tumor type, type of resection (if pulmonary resection is applied), and the patients’ general condition. In terms of the type of primary tumor, specifically when the primary tumor is breast cancer, a tissue diagnosis is recommended, since the first choice of treatment for metastatic breast cancer is systemic therapy (15). However, when the primary tumor is colorectal cancer (CRC), pulmonary resection can be the first choice, as PM is recommended for resectable distant metastases from CRC (16).
The combination of radiological findings, smoking history, and type of previous malignancy can reportedly improve the ability to predict primary lung cancer in the presence of a solitary pulmonary lesion that appears after treatment for a previous malignancy (17). The actual frequency of the diagnosis of SPN is also useful information. The reported frequencies of pulmonary metastases from a primary tumor and lung cancer in pulmonary nodule(s) with a history of malignancy are shown in Table 1. The ratio of pulmonary metastases among pulmonary nodules in patients with previous malignancies was observed to range from 16% to 62%. In cases of smoking-related cancers, such as esophageal cancer, head and neck cancer, and transitional cell carcinoma of the urinary tract, more than half of pulmonary nodules were primary lung cancer and were typically stage I. Based on these data, when a pulmonary nodule is detected in patients with a history of smoking-related cancer, surgery should be proactively indicated if the patient has a good general condition and can tolerate surgery. The differential diagnosis of SPN is often difficult in practical settings. In such situations, resection of SPN is considered beneficial (21).

Full table
Radiological examinations
The most important part of the preoperative evaluation for PM is the radiological examination. Its purposes include (I) the differential diagnosis of pulmonary nodules; (II) the evaluation of the precise number, location, and features of pulmonary nodules; and (III) the search for extrathoracic metastases. The standard modalities include thin-slice computed tomography (CT) and 18F-fluorodeoxyglucose positron emission tomography/CT (FDG-PET/CT) (22).
Thin-slice CT is useful for the differential diagnosis and evaluation of the precise number and location of lesions as well as the prediction of their pathological aggressiveness. The differential diagnosis of pulmonary metastases from primary lung cancer is an important issue. In general, pulmonary metastases tend to present as well-defined solid and round nodules without ground-glass opacity (GGO). Differential diagnosis by preoperative CT is often challenging in patients with a history of breast cancer and biliary tract cancer (23,24). In CRC, whether or not nodules have a GGO component have been reported to be useful in making a differential diagnosis (18). In contrast, GGO is not a suitable criterion for discriminating primary lung cancer from pulmonary metastases from breast cancer (25), pancreatic cancer (26), malignant melanoma (27), or transitional cell carcinoma of urinary tract (14). In cases with a small nodule (diameter <10 mm), discrimination of pulmonary metastases from a benign lesion are difficult. Previous studies have reported that in patients who underwent 2-mm slice thickness CT with a history of extrapulmonary malignancy, nodules smaller than 10 mm were more likely to be benign, whereas those greater than or equal to 10 mm were more likely to be malignant, while most nodules less than 10 mm from the pleura were benign (28). Thus, an evaluation by serial CT is important in patients with such nodules, and careful follow-up is needed (29).
Whether or not thoracic surgeons should palpate the lung during surgery remains controversial, as the sensitivity rate of high-resolution CT for detecting pulmonary metastases has been reported to be 75% (30). It is important to note that the ratio of small pulmonary metastases varies among tumor types. Pulmonary metastases from osteosarcoma tends to be small. Thus, the sensitivity of high-resolution CT for detecting pulmonary metastases is lower than that of other tumor types (31-33). Some authors recommend sufficient palpation during surgery in order to avoid missing small metastatic nodules in patients with pulmonary metastases from osteosarcoma, as preoperative CT may underestimate the number of metastatic lesions (32,34). However, the sensitivity of high-resolution CT for detecting pulmonary metastases in patients with non-osteosarcoma is sufficiently high (31), so the necessity of palpating the lung during surgery remains controversial (35,36). Because sufficient palpation of the lung via the video-assisted thoracoscopic surgery (VATS) approach is often difficult, this issue is often replaced by a discussion of whether or not thoracic surgeons should perform thoracotomy at surgery. Although thoracotomy allows for the manual palpation of the ipsilateral hemithorax and, in some cases, may be superior to a VATS approach for radical resection, the impact of non-resected pulmonary metastases on patient survival has not been clearly evaluated (30). Recently, this issue was addressed in patients with CRC. Murakawa et al. (37) reported that thoracoscopic metastasectomy was associated with a better overall survival than an open approach in a cohort of 1,047 patients. They then concluded that, in terms of tumor identification and survival outcome, the thoracoscopic approach might be acceptable for resection of pulmonary metastases in the current era.
In addition to its utility for the differential diagnosis and evaluation of the number and location of pulmonary nodules, CT is also useful in predicting pathologic findings. The morphologic features of aerogenous spread of floating cancer (AFSC) cell clusters and vascular invasion at the pulmonary metastases in CRC have been reported as prognostic factors after PM (38,39). Welter et al. (40) reported that pulmonary metastases from CRC and other epithelial tumors were associated with a higher rate of having AFSC than melanoma, renal cell carcinoma (RCC), and sarcoma. Recently, Issa et al. (41) compared the radiomorphology and microscopic growth characteristics of 232 pulmonary metastases to evaluate the presence of aggressive patterns of local intrapulmonary dissemination. In this study, they drew the important conclusion that the radiomorphologic characteristics of lung metastases corresponded well with the microscopic appearance of the resected lesion. Thoracic surgeons should be aware of these radiomorphologic findings, as resection with a sufficient margin should be performed when treating tumors with microscopic aggressive patterns.
When PM is planned, the existence of extrathoracic metastases should be assessed in cooperation with the doctor who treated the primary tumor. Additionally, brain magnetic resonance imaging or CT should be performed in consideration of the possibility of brain metastases, depending on the primary tumor type. Although no data exists on the superiority of FDG-PET/CT to thin-slice CT in terms of sensitivity for detecting pulmonary metastases, FDG-PET/CT is useful and regarded as the standard examination for staging among various cancer types to search for extrathoracic metastases (22,42-44). In many tumor types, FDG-PET/CT showed a higher diagnostic accuracy than PET or CT alone for detecting tumor recurrence. Thus, it is more appropriate to use for patient selection for PM than other conventional imaging modalities (45-47). Recently, it was reported that dual-time point FDG-PET/CT could be useful for distinguishing primary and metastatic lung adenocarcinoma in patients with SPN (48).
Thin-slice CT and FDG-PET/CT can also be used to evaluate mediastinal staging. However, information on the diagnostic ability of thin-slice CT or FDG-PET/CT for mediastinal lymph node (LN) metastases from pulmonary metastases remains limited. This is attributed to the following: if mediastinal LN metastases is strongly suggested by preoperative imaging examinations, surgery is often avoided, so the pathologic diagnosis of the mediastinal LN cannot be done. Winter et al. reported that preoperative CT had 84% sensitivity and 97% specificity for predicting LN metastases in pulmonary metastases from RCC (49). In contrast, the sensitivities of preoperative radiologic examinations for LN metastases were found to be relatively low in patients with metastatic CRC; the sensitivities of positive PET, a bulky LN on CT, and both were 35%, 25%, and 23%, respectively, and the specificities were 96%, 93%, and 97%, respectively (50). Additionally, the sensitivity of preoperative radiological examinations for LN metastases may differ among cancer types. Further data collection is needed concerning this issue. Endobronchial ultrasound guided transbronchial needle aspiration (EBUS‐TBNA) has also been reported as an efficient modality for screening mediastinal LNs/masses for malignancy in patients with extrapulmonary malignancies (51). Based on the findings of thin-slice CT and FDG-PET/CT, EBUS-TBNA should be considered in each case.
Thus, Mediastinal LN dissection, or at least sampling during surgery, is recommended because many reports support the notion that mediastinal LN metastases are a significant negative prognostic factor for survival after PM (2,49,50,52,53).
Indications for PM
Eligibility criteria
The indication of PM is considered from both physiological and oncological points of view. The eligibility criteria of PM were first described by Thomford et al. in the 1960s (54), who reported that the indications for PM include control of the primary tumor, no other distant metastatic disease besides the lung, conditions that make surgery technically feasible, and sufficient cardiopulmonary function of the patient to tolerate surgery.
Since then, many advances in radiological assessments and surgical management approaches have been made, and the eligibility criteria for PM have been extensively modified. At present, the criteria summarized by Kondo et al. (55) are widely used (Table 2). One of the major criteria, “Pulmonary metastases are considered to be completely resectable,” is thought to be the most important, as it was observed that patients who undergo incomplete resection are associated with a poor outcome, regardless of the tumor type. The only case in which incomplete resection of pulmonary metastases is suitable is for the control of pneumothorax resulting from pulmonary metastases (56).
Thoracic surgeons should also pay close attention to whether the tumor is rapidly growing and/or spreading. A sufficient observation period is important when considering the indication for PM for patients with a high risk of recurrence, such as those with rapidly growing pulmonary metastases, multiple pulmonary metastases, a short disease interval, or a history of distant metastases other than in the lung. Observation for three months with or without chemotherapy after the first detection of pulmonary metastases is recommended in order to decide the indication of PM. A delayed operation seems justified if the indication for resection is questionable as no evidence has indicated that a longer interval between the detection of pulmonary metastases and PM worsens the outcomes of patients who undergo PM (57,58). Having an optimal observation period provides further information on the prognosis of the patient. Patients with pulmonary metastatic tumors that have a short doubling time have been reported to be associated with worse prognosis than those with a long doubling time in various tumor types (59-63). Furthermore, patients with osteosarcoma who develop pulmonary metastases during chemotherapy have also shown worse survival rates than those who develop pulmonary metastases in the period without chemotherapy (64).
In addition to the criteria described above, it is also worth to consider whether patients have poor prognostic factors. There have been many reports on prognostic factors after PM in various tumor types (2). Having poor prognostic factors does not necessarily mean that the patient is not indicated for PM; however, in general, the indication should be carefully considered when patients have multiple poor prognostic factors, as these patients have a high probability of developing recurrence and would benefit more from systemic treatment than surgery.
Prognostic factors for each tumor type
CRC
CRC is the most common primary tumor in patients who undergo PM, followed by RCC, breast cancer, otorhinolaryngological cancer, and uterine malignancies (65). Numerous reports on the prognostic factors for PM for CRC are available (66-68). A general systemic review of PM for CRC has been discussed elsewhere (69). Noteworthy recent reports are therefore discussed in this section. In recent years, the survival prognosis after PM in patients with CRC has improved remarkably (70,71). Newer chemotherapy regimens may have played a positive impact on these patients (71). In this clinical context, the prognostic factors after PM in patients with CRC should be evaluated based on recent data.
The poor prognostic factors identified by a multivariate analysis using a large cohort include the tumor number (72,73), tumor size (72), preoperative serum carcinoembryonic antigen (CEA) level (72,73), LN metastases (72), and completeness of resection (72). In addition, an age that is 70 years or older, a disease-free interval (DFI) of less than 2 years, and extrathoracic metastatic lesions treated curatively before PM resection have also reported as poor prognostic factors (73). Regarding patients with multiple metastases, Maniwa et al. (74), who analyzed 247 patients with multiple metastases, found that heterogeneity, defined as the difference between the maximum and minimum tumor diameter exceeding 5 mm, may be a prognostic indicator. In their report, heterogeneity, >five metastases, a high preoperative serum CEA level, and a DFI of <2 years were identified as prognostic factors among patients with multiple metastases. Whether or not a history of hepatic metastases is a prognostic factor remains controversial. A recent meta-analysis showed that a history of hepatic metastases worsens the prognosis. In contrast, in a report involving the largest cohort from Japan, a history of hepatic metastases were not a prognostic factor according to a multivariate analysis (72), and many reports from single institutions support this result (75-79). Currently, it is believed that PM remains a viable treatment option in patients with a history of hepatic metastases; however, stratification of patients who benefit from PM among these patients is necessary. Shimizu et al. (80) recently analyzed the outcomes of PM in patients with a history of liver metastases and showed that a high preoperative CEA level was an independent prognostic factor for the overall survival. Although there have been only a few reports on PM for patients with both pulmonary and hepatic metastases detected simultaneously with the primary tumor, PM for such patients was still shown to provide favorable long-term outcomes (81,82). However, a history of distant metastases other than to the liver is believed to be a poor prognostic factor (72,79). Thus, the indication of PM for such patients should be carefully considered.
RCC
RCC is the second-most common primary tumor in patients who undergo PM (65,83). Reports on PM for RCC published after 2001 are shown in Table 3 (49,84-89,91-98). The factors associated with prognosis that suggested a poor overall survival that were shared among multiple reports included incomplete resection, a large number of metastases, a large size of metastases, and a short DFI. In the clinical guideline for RCC in Japan, PM is recommended in cases with a good performance status, longer DFI, and good possibility of complete resection (99). Given marked advances in immunotherapy and molecular-targeted therapy in recent years (100,101), the further accumulation of data in the current era is needed.
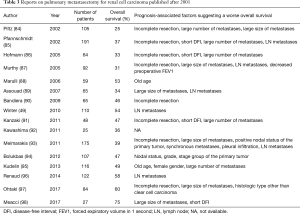
Full table
Head and neck cancer
Squamous cell carcinoma is the most common histological type of head and neck cancer, and the utility of PM for these cancers has been reviewed elsewhere (102,103). Adenoid cystic carcinomas have been associated with a better prognosis than head and neck squamous cell carcinoma (104). Reports on PM for head and neck cancer published after 2001 are shown in Table 4 (105-118). The factors associated with prognosis that suggested a poor overall survival and were shared among multiple reports included incomplete resection, a short DFI, an old age, oral cavity primary lesion, and recurrence before lung metastases.
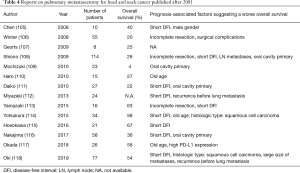
Full table
Uterine malignancies
Uterine malignancies include uterine corpus cancer and uterine cervical cancer. In terms of the histologic type, these cancers can emerge as either carcinoma or sarcoma. Although they are considered independent entities, PM for uterine malignancies is often discussed together due to the limited number of such patients. Bilancia et al. recently published a comprehensive review of this issue (119). Reports on PM for head and neck cancer published after 2001 are shown in Table 5 (120-126). The factors associated with prognosis that suggested a poor overall survival and were shared among multiple reports were a short DFI, cervix primary lesion, and a large number of metastases. However, a short DFI was only reported as a factor by three authors. Thus, careful consideration must be given for the indication of PM for patients with a short DFI.
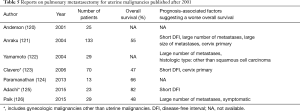
Full table
Conclusions
The indication of PM should be considered from both physiological and oncological points of view. In addition to the general eligibility criteria of PM, prognostic factors of each tumor type should be considered when deciding the indication of PM. When patients have multiple poor prognostic factors and/or a short DFI, thoracic surgeons should not hesitate to observe the patient for a certain period before deciding on the indication of PM. In the current era, when treatment options besides PM, such as SBRT, immunotherapy, and molecular-targeted therapy are becoming increasingly accessible, a multidisciplinary discussion is needed in order to decide the indication of PM.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Khosro Hekmat) for the series “Pulmonary Metastases” published in Journal of Thoracic Disease. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/jtd-19-3791). The series “Pulmonary Metastases” was commissioned by the editorial office without any funding or sponsorship. YS serves as an unpaid editorial board member of Journal of Thoracic Disease from Sep 2019 to Aug 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: All authors are accountable for all aspects of this work in ensuring that questions related to the accuracy or integrity of any part of this work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Barney JD, Churchill EJ. Adenocarcinoma of the kidney with metastases to the lung: cured nephrectomy and lobectomy. J Urol 1939;42:269-76. [Crossref]
- Higashiyama M, Tokunaga T, Nakagiri T, et al. Pulmonary metastasectomy: outcomes and issues according to the type of surgical resection. Gen Thorac Cardiovasc Surg 2015;63:320-30. [Crossref] [PubMed]
- Treasure T, Macbeth F. Is Surgery Warranted for Oligometastatic Disease? Thorac Surg Clin 2016;26:79-90. [Crossref] [PubMed]
- Ciombor KK, Wu C, Goldberg RM. Recent therapeutic advances in the treatment of colorectal cancer. Annu Rev Med 2015;66:83-95. [Crossref] [PubMed]
- Lodeweges JE, Klinkenberg TJ, Ubbels JF, et al. Long-term outcome of surgery or stereotactic radiotherapy for lung oligometastases. J Thorac Oncol 2017;12:1442-5. [Crossref] [PubMed]
- Cheung FP, Alam NZ, Wright GM. The past, present and future of pulmonary metastasectomy: a review article. Ann Thorac Cardiovasc Surg 2019;25:129-41. [Crossref] [PubMed]
- Lugg ST, Tikka T, Agostini PJ, et al. Smoking and timing of cessation on postoperative pulmonary complications after curative-intent lung cancer surgery. J Cardiothorac Surg 2017;12:52. [Crossref] [PubMed]
- Kanzaki R, Shintani Y, Inoue M, et al. Late-onset pulmonary fistula after resection of pulmonary metastases from colorectal cancer following perioperative chemotherapy with bevacizumab. Ann Thorac Cardiovasc Surg 2017;23:149-52. [Crossref] [PubMed]
- Nakahara K, Monden Y, Ohno K, et al. A method for predicting postoperative lung function and its relation to postoperative complications in patients with lung cancer. Ann Thorac Surg 1985;39:260-5. [Crossref] [PubMed]
- Sawabata N, Nagayasu T, Kadota Y, et al. Risk assessment of lung resection for lung cancer according to pulmonary function: republication of systematic review and proposals by guideline committee of the Japanese association for chest surgery 2014. Gen Thorac Cardiovasc Surg 2015;63:14-21. [Crossref] [PubMed]
- Ha D, Mazzone PJ, Ries AL, et al. The utility of exercise testing in patients with lung cancer. J Thorac Oncol 2016;11:1397-410. [Crossref] [PubMed]
- Kanzaki R, Kimura T, Kawamura T, et al. Surgery for malignant pulmonary nodules in patients with a history of oesophageal cancer. Interact Cardiovasc Thorac Surg 2017;24:418-24. [PubMed]
- Kanzaki R, Inoue M, Minami M, et al. Surgery for pulmonary malignancies in patients with a previous history of head and neck squamous cell carcinoma. Surg Today 2014;44:646-52. [Crossref] [PubMed]
- Kanzaki R, Kanou T, Ose N, et al. Surgery for solitary pulmonary nodule occurred in patients with a history of transitional cell carcinoma of the urinary tract. Haigan 2019;59:201. Abstract in Japanese.
- The Japanese Breast Cancer Society. Clinical practice guideline for breast cancer 2018. Tokyo: Kanehara, 2018.
- Hashiguchi Y, Muro K, Saito Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol 2020;25:1-42. [Crossref] [PubMed]
- Nakadate A, Nakadate M, Sato Y, et al. Predictors of primary lung cancer in a solitary pulmonary lesion after a previous malignancy. Gen Thorac Cardiovasc Surg 2017;65:698-704. [Crossref] [PubMed]
- Ohtaki Y, Shimizu K, Nagashima T, et al. Clinical and radiological discrimination of solitary pulmonary lesions in colorectal cancer patients. World J Surg 2018;42:1161-70. [Crossref] [PubMed]
- Kinoshita T, Yoshida J, Ishii G, et al. The availability of pre- and intraoperative evaluation of a solitary pulmonary nodule in breast cancer patients. Ann Thorac Cardiovasc Surg 2015;21:31-6. [Crossref] [PubMed]
- Loizzi M, Sollitto F, Sardelli P, et al. Endothoracic nodules in patients who under-went nephrectomy for renal cell carcinoma. Results of surgical resection. Minerva Med 2003;94:103-10. [PubMed]
- Sakamoto M, Murakawa T, Kitano K, et al. Resection of solitary pulmonary lesion is beneficial to patients with a history of malignancy. Ann Thorac Surg 2010;90:1766-71. [Crossref] [PubMed]
- Detterbeck FC, Grodzki T, Gleeson F, Robert JH. Imaging requirements in the practice of pulmonary metastasectomy. J Thorac Oncol 2010;5:S134-9. [Crossref] [PubMed]
- Zhu L, Bian H, Yang L, et al. 18Fluorodeoxyglucose-positron emission tomography/computed tomography features of suspected solitary pulmonary lesions in breast cancer patients following previous curative treatment. Thorac Cancer 2019;10:1086-95. [Crossref] [PubMed]
- Kawaguchi K, Taniguchi T, Fukui T, et al. Radiological findings and surgical outcomes of pulmonary metastases originating from biliary tract carcinoma. Gen Thorac Cardiovasc Surg 2019;67:962-8. [Crossref] [PubMed]
- Tanaka K, Shimizu K, Ohtaki Y, et al. Diagnosis and surgical resection of solitary pulmonary nodules in patients with breast cancer. Mol Clin Oncol 2013;1:117-23. [Crossref] [PubMed]
- Gaeta M, Volta S, Scribano E, et al. Air-space pattern in lung metastases from adenocarcinoma of the GI tract. J Comput Assist Tomogr 1996;20:300-4. [Crossref] [PubMed]
- Okita R, Yamashita M, Nakata M, et al. Multiple ground-glass opacity in metastases of malignant melanoma diagnosed by lung biopsy. Ann Thorac Surg 2005;79:e1-2. [Crossref] [PubMed]
- Hanamiya M, Aoki T, Yamashita Y, et al. Frequency and significance of pulmonary nodules on thin-section CT in patients with extrapulmonary malignant neoplasms. Eur J Radiol 2012;81:152-7. [Crossref] [PubMed]
- Nakamura T, Matsumine A, Matsusaka M, et al. Analysis of pulmonary nodules in patients with high-grade soft tissue sarcomas. PLoS One 2017;12:e0172148 [Crossref] [PubMed]
- Macherey S, Doerr F, Heldwein M, et al. Is manual palpation of the lung necessary in patients undergoing pulmonary metastasectomy? Interact Cardiovasc Thorac Surg 2016;22:351-9. [Crossref] [PubMed]
- Kang MC, Kang CH, Lee HJ, et al. Accuracy of 16-channel multi-detector row chest computed tomography with thin sections in the detection of metastatic pulmonary nodules. Eur J Cardiothorac Surg 2008;33:473-9. [Crossref] [PubMed]
- Waters DJ, Coakley FV, Cohen MD, et al. The detection of pulmonary metastases by helical CT: a clinicopathologic study in dogs. J Comput Assist Tomogr 1998;22:235-40. [Crossref] [PubMed]
- Kayton ML, Huvos AG, Casher J, et al. Computed tomographic scan of the chest underestimates the number of metastatic lesions in osteosarcoma. J Pediatr Surg 2006;41:200-6; discussion 200-6. [Crossref] [PubMed]
- Gao E, Li Y, Zhao W, et al. Necessity of thoracotomy in pulmonary metastases of osteosarcoma. J Thorac Dis 2019;11:3578-83. [Crossref] [PubMed]
- Nakajima J, Murakawa T, Fukami T, et al. Is finger palpation at operation indispensable for pulmonary metastasectomy in colorectal cancer? Ann Thorac Surg 2007;84:1680-4. [Crossref] [PubMed]
- Nakas A, Klimatsidas MN, Entwisle J, et al. Video-assisted versus open pulmonary metastasectomy: the surgeon’s finger or the radiologist’s eye? Eur J Cardiothorac Surg 2009;36:469-74. [Crossref] [PubMed]
- Murakawa T, Sato H, Okumura S, et al. Thoracoscopic surgery versus open surgery for lung metastases of colorectal cancer: a multi-institutional retrospective analysis using propensity score adjustment dagger. Eur J Cardiothorac Surg 2017;51:1157-63. [Crossref] [PubMed]
- Shiono S, Ishii G, Nagai K, et al. Predictive factors for local recurrence of resected colorectal lung metastases. Ann Thorac Surg 2005;80:1040-5. [Crossref] [PubMed]
- Shiono S, Ishii G, Nagai K, et al. Histopathologic prognostic factors in resected colorectal lung metastases. Ann Thorac Surg 2005;79:278-82; discussion 83. [Crossref] [PubMed]
- Welter S, Arfanis E, Christoph D, et al. Growth patterns of pulmonary metastases: should we adjust resection techniques to primary histology and size? Eur J Cardiothorac Surg 2017;52:39-46. [Crossref] [PubMed]
- Issa N, Arfanis E, Hager T, et al. A prospective comparison of growth patterns with radiomorphology in 232 lung metastases-basis for patient tailored resection planning? J Thorac Dis 2019;11:2822-31. [Crossref] [PubMed]
- Fortes DL, Allen MS, Lowe VJ, et al. The sensitivity of 18F-fluorodeoxyglucose positron emission tomography in the evaluation of metastatic pulmonary nodules. Eur J Cardiothorac Surg 2008;34:1223-7. [Crossref] [PubMed]
- Pastorino U, Veronesi G, Landoni C, et al. Fluorodeoxyglucose positron emission tomography improves preoperative staging of resectable lung metastases. J Thorac Cardiovasc Surg 2003;126:1906-10. [Crossref] [PubMed]
- Hagi T, Nakamura T, Sugino Y, et al. Is FDG-PET/CT useful for diagnosing pulmonary metastases in patients with soft tissue sarcoma? Anticancer Res 2018;38:3635-9. [Crossref] [PubMed]
- Kitajima K, Murakami K, Yamasaki E, et al. Performance of FDG-PET/CT for diagnosis of recurrent uterine cervical cancer. Eur Radiol 2008;18:2040-7. [Crossref] [PubMed]
- Chung HH, Jo H, Kang WJ, et al. Clinical impact of integrated PET/CT on the management of suspected cervical cancer recurrence. Gynecol Oncol 2007;104:529-34. [Crossref] [PubMed]
- Lee JY, Yoon SM, Kim JT, et al. Diagnostic and prognostic value of preoperative 18F-fluorodeoxyglucose positron emission tomography/computed tomography for colorectal cancer: comparison with conventional computed tomography. Intest Res 2017;15:208-14. [Crossref] [PubMed]
- Pahk K, Chung JH, Kim S, et al. Predictive value of dual-time 18F-FDG PET/CT to distinguish primary lung and metastatic adenocarcinoma in solitary pulmonary nodule. Tumori 2018;104:207-12. [Crossref] [PubMed]
- Winter H, Meimarakis G, Angele MK, et al. Tumor infiltrated hilar and mediastinal lymph nodes are an independent prognostic factor for decreased survival after pulmonary metastasectomy in patients with renal cell carcinoma. J Urol 2010;184:1888-94. [Crossref] [PubMed]
- Hamaji M, Cassivi SD, Shen KR, et al. Is lymph node dissection required in pulmonary metastasectomy for colorectal adenocarcinoma? Ann Thorac Surg 2012;94:1796-800. [Crossref] [PubMed]
- Nambirajan A, Longchar M, Madan K, et al. Endobronchial ultrasound-guided transbronchial needle aspiration cytology in patients with known or suspected extra-pulmonary malignancies: A cytopathology-based study. Cytopathology 2019;30:82-90. [Crossref] [PubMed]
- Shiono S, Matsutani N, Okumura S, et al. The prognostic impact of lymph-node dissection on lobectomy for pulmonary metastases. Eur J Cardiothorac Surg 2015;48:616-21; discussion 21. [Crossref] [PubMed]
- Ali K, Cho S, Jang HJ, et al. Predictive factors of thoracic lymph node metastases accompanying pulmonary metastases from colorectal cancer. Thorac Cardiovasc Surg 2019;67:683-7. [Crossref] [PubMed]
- Thomford NR, Woolner LB, Clagett OT. The surgical treatment of metastatic tumors in the lungs. J Thorac Cardiovasc Surg 1965;49:357-63. [Crossref] [PubMed]
- Kondo H, Okumura T, Ohde Y, et al. Surgical treatment for metastatic malignancies. Pulmonary metastases: indications and outcomes. Int J Clin Oncol 2005;10:81-5. [Crossref] [PubMed]
- Hoag JB, Sherman M, Fasihuddin Q, et al. A comprehensive review of spontaneous pneumothorax complicating sarcoma. Chest 2010;138:510-8. [Crossref] [PubMed]
- Tanaka Y, Maniwa Y, Nishio W, et al. The optimal timing to resect pulmonary metastases. Eur J Cardiothorac Surg 2008;33:1135-8. [Crossref] [PubMed]
- Krüger M, Schmitto JD, Wiegmann B, et al. Optimal timing of pulmonary metastasectomy--is a delayed operation beneficial or counterproductive? Eur J Surg Oncol 2014;40:1049-55. [Crossref] [PubMed]
- Ollila DW, Stern SL, Morton DL. Tumor doubling time: a selection factor for pulmonary resection of metastatic melanoma. J Surg Oncol 1998;69:206-11. [Crossref] [PubMed]
- Tomimaru Y, Noura S, Ohue M, et al. Metastatic tumor doubling time is an independent predictor of intrapulmonary recurrence after pulmonary resection of solitary pulmonary metastases from colorectal cancer. Dig Surg 2008;25:220-5. [Crossref] [PubMed]
- Kobayashi Y, Fukui T, Ito S, et al. Pulmonary metastasectomy for gastric cancer: a 13-year single-institution experience. Surg Today 2013;43:1382-9. [Crossref] [PubMed]
- Kawaguchi K, Uehara K, Nakayama G, et al. Growth rate of chemotherapy-naive lung metastases from colorectal cancer could be a predictor of early relapse after lung resection. Int J Clin Oncol 2016;21:329-34. [Crossref] [PubMed]
- Nakamura T, Matsumine A, Takao M, et al. Impact of tumor volume doubling time on post-metastatic survival in bone or soft-tissue sarcoma patients treated with metastasectomy and/or radiofrequency ablation of the lung. Onco Targets Ther 2017;10:559-64. [Crossref] [PubMed]
- Ahmed G, Zamzam M, Kamel A, et al. Effect of timing of pulmonary metastases occurrence on the outcome of metastasectomy in osteosarcoma patients. J Pediatr Surg 2019;54:775-9. [Crossref] [PubMed]
- Shimizu H, Endo S, Natsugoe S, et al. Thoracic and cardiovascular surgery in Japan in 2016: annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2019;67:377-411. [Crossref] [PubMed]
- Saito Y, Omiya H, Kohno K, et al. Pulmonary metastasectomy for 165 patients with colorectal carcinoma: A prognostic assessment. J Thorac Cardiovasc Surg 2002;124:1007-13. [Crossref] [PubMed]
- Inoue M, Ohta M, Iuchi K, et al. Benefits of surgery for patients with pulmonary metastases from colorectal carcinoma. Ann Thorac Surg 2004;78:238-44. [Crossref] [PubMed]
- Higashiyama M, Kodama K, Higaki N, et al. Surgery for pulmonary metastases from colorectal cancer: the importance of prethoracotomy serum carcinoembryonic antigen as an indicator of prognosis. Jpn J Thorac Cardiovasc Surg 2003;51:289-96. [Crossref] [PubMed]
- Phillips JD, Hasson RM. Surgical management of colorectal lung metastases. J Surg Oncol 2019;119:629-35. [Crossref] [PubMed]
- Kanzaki R, Inoue M, Kimura T, et al. Role of pulmonary metastasectomy in colorectal cancer in the era of modern multidisciplinary therapy. Surg Today 2017;47:1111-8. [Crossref] [PubMed]
- Nakajima J, Iida T, Okumura S, et al. Recent improvement of survival prognosis after pulmonary metastasectomy and advanced chemotherapy for patients with colorectal cancer. Eur J Cardiothorac Surg 2017;51:869-73. [Crossref] [PubMed]
- Iida T, Nomori H, Shiba M, et al. Prognostic factors after pulmonary metastasectomy for colorectal cancer and rationale for determining surgical indications: a retrospective analysis. Ann Surg 2013;257:1059-64. [Crossref] [PubMed]
- Okumura T, Boku N, Hishida T, et al. Surgical outcome and prognostic stratification for pulmonary metastases from colorectal cancer. Ann Thorac Surg 2017;104:979-87. [Crossref] [PubMed]
- Maniwa T, Mori K, Ohde Y, et al. Heterogeneity of Tumor Sizes in Multiple Pulmonary Metastases of Colorectal Cancer as a Prognostic Factor. Ann Thorac Surg 2017;103:254-60. [Crossref] [PubMed]
- Chen F, Shoji T, Sakai H, et al. Lung metastasectomy for colorectal carcinoma in patients with a history of hepatic metastases. Ann Thorac Cardiovasc Surg 2011;17:13-8. [Crossref] [PubMed]
- Ihn MH, Kim DW, Cho S, et al. Curative resection for metachronous pulmonary metastases from colorectal cancer: analysis of survival rates and prognostic factors. Cancer Res Treat 2017;49:104-15. [Crossref] [PubMed]
- Kim S, Kim HK, Cho JH, et al. Prognostic factors after pulmonary metastasectomy of colorectal cancers: influence of liver metastases. World J Surg Oncol 2016;14:201. [Crossref] [PubMed]
- Suzuki H, Kiyoshima M, Kitahara M, et al. Long-term outcomes after surgical resection of pulmonary metastases from colorectal cancer. Ann Thorac Surg 2015;99:435-40. [Crossref] [PubMed]
- Kanzaki R, Ose N, Kawamura T, et al. Does history of liver metstasis affects long-term outcomes of pulmonary metastasectomy for colorectal canccer? Abstracts of annual 118th congress of Japan Surgical Society. Tokyo2016: Japan Surgical Society p 1258 Abstract in Japanese; 2018.
- Shimizu K, Ohtaki Y, Okumura T, et al. Outcomes and prognostic factors after pulmonary metastasectomy in patients with colorectal cancer with previously resected hepatic metastases. J Thorac Cardiovasc Surg 2019;157:2049-57.e1. [Crossref] [PubMed]
- Nozawa H, Tanaka J, Nishikawa T, et al. Predictors and outcome of complete removal of colorectal cancer with synchronous lung metastases. Mol Clin Oncol 2015;3:1041-7. [Crossref] [PubMed]
- Stelzner S, Radulova-Mauersberger O, Zschuppe E, et al. Prognosis in patients with synchronous colorectal cancer metastases after complete resection of the primary tumor and the metastases. J Surg Oncol 2019;120:438-45. [Crossref] [PubMed]
- Zhao Y, Li J, Li C, et al. Prognostic factors for overall survival after lung metastasectomy in renal cell cancer patients: A systematic review and meta-analysis. Int J Surg 2017;41:70-7. [Crossref] [PubMed]
- Piltz S, Meimarakis G, Wichmann MW, et al. Long-term results after pulmonary resection of renal cell carcinoma metastases. Ann Thorac Surg 2002;73:1082-7. [Crossref] [PubMed]
- Pfannschmidt J, Hoffmann H, Muley T, et al. Prognostic factors for survival after pulmonary resection of metastatic renal cell carcinoma. Ann Thorac Surg 2002;74:1653-7. [Crossref] [PubMed]
- Hofmann HS, Neef H, Krohe K, et al. Prognostic factors and survival after pulmonary resection of metastatic renal cell carcinoma. Eur Urol 2005;48:77-81; discussion 81. Discussion. [Crossref] [PubMed]
- Murthy SC, Kim K, Rice TW, et al. Can we predict long-term survival after pulmonary metastasectomy for renal cell carcinoma? Ann Thorac Surg 2005;79:996-1003. [Crossref] [PubMed]
- Marulli G, Sartori F, Bassi PF, et al. Long-term results of surgical management of pulmonary metastases from renal cell carcinoma. Thorac Cardiovasc Surg 2006;54:544-7. [Crossref] [PubMed]
- Assouad J, Petkova B, Berna P, et al. Renal cell carcinoma lung metastases surgery: pathologic findings and prognostic factors. Ann Thorac Surg 2007;84:1114-20. [Crossref] [PubMed]
- Bandiera A, Melloni G, Freschi M, et al. Prognostic factors and analysis of S100a4 protein in resected pulmonary metastases from renal cell carcinoma. World J Surg 2009;33:1414-20. [Crossref] [PubMed]
- Kanzaki R, Higashiyama M, Fujiwara A, et al. Long-term results of surgical resection for pulmonary metastases from renal cell carcinoma: a 25-year single-institution experience. Eur J Cardiothorac Surg 2011;39:167-72. [Crossref] [PubMed]
- Kawashima A, Nakayama M, Oka D, et al. Pulmonary metastasectomy in patients with renal cell carcinoma: a single-institution experience. Int J Clin Oncol 2011;16:660-5. [Crossref] [PubMed]
- Meimarakis G, Angele M, Staehler M, et al. Evaluation of a new prognostic score (Munich score) to predict long-term survival after resection of pulmonary renal cell carcinoma metastases. Am J Surg 2011;202:158-67. [Crossref] [PubMed]
- Bölükbas S, Kudelin N, Eberlein M, et al. The influence of the primary tumor on the long-term results of pulmonary metastasectomy for metastatic renal cell carcinoma. Thorac Cardiovasc Surg 2012;60:390-7. [PubMed]
- Kudelin N, Bölükbas S, Eberlein M, et al. Metastasectomy with standardized lymph node dissection for metastatic renal cell carcinoma: an 11-year single-center experience. Ann Thorac Surg 2013;96:265-70; discussion 270-1. [Crossref] [PubMed]
- Renaud S, Falcoz PE, Alifano M, et al. Systematic lymph node dissection in lung metastasectomy of renal cell carcinoma: an 18 years of experience. J Surg Oncol 2014;109:823-9. [Crossref] [PubMed]
- Ohtaki Y, Shimizu K, Aokage K, et al. Histology is a prognostic indicator after pulmonary metastasectomy from renal cell carcinoma. World J Surg 2017;41:771-9. [Crossref] [PubMed]
- Meacci E, Nachira D, Congedo MT, et al. Lung metastasectomy following kidney tumors: outcomes and prognostic factors from a single-center experience. J Thorac Dis 2017;9:S1267-72. [Crossref] [PubMed]
- The Japanese Urological Association. Clinical practice guideline for renal cancer. Clinical practice guideline for renal Cancer 2017. Tokyo: medical review, 2017.
- Morse MA. Immunotherapy for resected pulmonary metastases. Thorac Surg Clin 2016;26:69-78. [Crossref] [PubMed]
- Posadas EM, Limvorasak S, Figlin RA. Targeted therapies for renal cell carcinoma. Nat Rev Nephrol 2017;13:496-511. [Crossref] [PubMed]
- Young ER, Diakos E, Khalid-Raja M, et al. Resection of subsequent pulmonary metastases from treated head and neck squamous cell carcinoma: systematic review and meta-analysis. Clin Otolaryngol 2015;40:208-18. [Crossref] [PubMed]
- Subramaniam NR, Reddy R, Balasubramanian D, et al. Is pulmonary metastasectomy beneficial in head and neck squamous cell carcinoma? A review of literature. Indian J Cancer 2017;54:2-5. [Crossref] [PubMed]
- Girelli L, Locati L, Galeone C, et al. Lung metastasectomy in adenoid cystic cancer: is it worth it? Oral Oncol 2017;65:114-8. [Crossref] [PubMed]
- Chen F, Sonobe M, Sato K, et al. Pulmonary resection for metastatic head and neck cancer. World J Surg 2008;32:1657-62. [Crossref] [PubMed]
- Winter H, Meimarakis G, Hoffmann G, et al. Does surgical resection of pulmonary metastases of head and neck cancer improve survival? Ann Surg Oncol 2008;15:2915-26. [Crossref] [PubMed]
- Geurts TW, Balm AJ, van Velthuysen ML, et al. Survival after surgical resection of pulmonary metastases and second primary squamous cell lung carcinomas in head and neck cancer. Head Neck 2009;31:220-6. [Crossref] [PubMed]
- Shiono S, Kawamura M, Sato T, et al. Pulmonary metastasectomy for pulmonary metastases of head and neck squamous cell carcinomas. Ann Thorac Surg 2009;88:856-60. [Crossref] [PubMed]
- Mochizuki T, Okumura S, Ishii G, et al. Surgical resection for oral tongue cancer pulmonary metastases. Interact Cardiovasc Thorac Surg 2010;11:56-9. [Crossref] [PubMed]
- Haro A, Yano T, Yoshida T, et al. Results of a surgical resection of pulmonary metastases from malignant head and neck tumor. Interact Cardiovasc Thorac Surg 2010;10:700-3. [Crossref] [PubMed]
- Daiko H, Nagai K, Yoshida J, et al. The role of pulmonary resection in tumors metastatic from head and neck carcinomas. Jpn J Clin Oncol 2010;40:639-44. [Crossref] [PubMed]
- Miyazaki T, Hasegawa Y, Hanai N, et al. Survival impact of pulmonary metastasectomy for patients with head and neck cancer. Head Neck 2013;35:1745-51. [Crossref] [PubMed]
- Yamazaki K, Shodo R, Ueki Y, et al. Therapeutic outcome after resection of pulmonary metastases from head and neck carcinomas. Indian J Otolaryngol Head Neck Surg 2015;67:124-8. [Crossref] [PubMed]
- Yotsukura M, Kinoshita T, Kohno M, et al. Survival predictors after resection of lung metastases of head or neck cancers. Thorac Cancer 2015;6:579-83. [Crossref] [PubMed]
- Hosokawa S, Funai K, Sugiyama K, et al. Survival outcomes after surgical resection of pulmonary metastases of head and neck tumours. J Laryngol Otol 2016;130:291-5. [Crossref] [PubMed]
- Nakajima Y, Iijima Y, Kinoshita H, et al. Surgical treatment for pulmonary metastases of head and neck cancer: study of 58 cases. Ann Thorac Cardiovasc Surg 2017;23:169-74. [Crossref] [PubMed]
- Okada S, Itoh K, Ishihara S, et al. Significance of PD-L1 expression in pulmonary metastases from head and neck squamous cell carcinoma. Surg Oncol 2018;27:259-65. [Crossref] [PubMed]
- Oki T, Hishida T, Yoshida J, et al. Survival and prognostic factors after pulmonary metastasectomy of head and neck cancer: what are the clinically informative prognostic indicators? Eur J Cardiothorac Surg 2019;55:942-7. [Crossref] [PubMed]
- Bilancia R, Nardini M, Waller D. Pulmonary metastasectomy in uterine malignancy: outcomes and prognostic factors. J Thorac Dis 2017;9:S1316-21. [Crossref] [PubMed]
- Anderson TM, McMahon JJ, Nwogu CE, et al. Pulmonary resection in metastatic uterine and cervical malignancies. Gynecol Oncol 2001;83:472-6. [Crossref] [PubMed]
- Anraku M, Yokoi K, Nakagawa K, et al. Pulmonary metastases from uterine malignancies: results of surgical resection in 133 patients. J Thorac Cardiovasc Surg 2004;127:1107-12. [Crossref] [PubMed]
- Yamamoto K, Yoshikawa H, Shiromizu K, et al. Pulmonary metastasectomy for uterine cervical cancer: a multivariate analysis. Ann Thorac Surg 2004;77:1179-82. [Crossref] [PubMed]
- Clavero JM, Deschamps C, Cassivi SD, et al. Gynecologic cancers: factors affecting survival after pulmonary metastasectomy. Ann Thorac Surg 2006;81:2004-7. [Crossref] [PubMed]
- Paramanathan A, Wright G. Pulmonary metastasectomy for sarcoma of gynaecologic origin. Heart Lung Circ 2013;22:270-5. [Crossref] [PubMed]
- Adachi M, Mizuno M, Mitsui H, et al. The prognostic impact of pulmonary metastasectomy in recurrent gynecologic cancers: a retrospective single-institution study. Nagoya J Med Sci 2015;77:363-72. [PubMed]
- Paik ES, Yoon A, Lee YY, et al. Pulmonary metastasectomy in uterine malignancy: outcomes and prognostic factors. J Gynecol Oncol 2015;26:270-6. [Crossref] [PubMed]



