
Prognostic relevance and clinical features of papillary muscle infarction with mitral regurgitation in patients with ST segment elevation myocardial infarction
Introduction
pPCI is the first treatment in patients with STEMI, despite advances in coronary reperfusion, papillary muscle infarction (PapMI) can result in dysfunction of the papillary muscles and subsequent ischemic functional mitral valve regurgitation (MR). MR occurs in 30–50% of cases after ST-segment elevation myocardial infarction (STEMI) and is independently associated with increased mortality, few reports assessed the Prognostic relevance and clinical features of patients with PapMI only (1-3). However, the precise clinical characteristics of PapMI with MR in patients with STEMI after primary percutaneous coronary intervention (pPCI) are currently under debate, and data on the prognostic significance of PapMI are not clear, partly because PapMI is difficult to verify or exclude by traditional imaging techniques such as echocardiography or myocardial perfusion imaging (SPECT). Late gadolinium-enhanced (LGE) cardiac magnetic resonance (CMR) imaging, typically using an inversion-recovery gradient echo sequence 10–30 min after intravenous injection of a gadolinium-based contrast agent (4-6), allows for the identification and accurate quantitative analysis of MI (7,8). Thus, our aims of this study were to determine the clinical features and prognosis of PapMI with MR in patients with STEMI detected by CMR. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-3476).
Methods
Population
The population was composed of consecutive patients with first STEMI enrolled in a prospective study between June 2018 and February 2020 at Drum Tower Hospital affiliated to Medical School of Nanjing University and Xinxiang Central Hospital. We used the Sample size formula to calculate the Sample size required for the study before it stared. The definition of STEMI was based on the ESC/ACCF/AHA/WHF consensus document, and was established by the presence of typical chest pain lasting >30 minutes, sustained 1.0 mm ST-segment elevation in at least 2 contiguous leads on ECG, and cardiac enzyme elevation. According to the results of CMR and echocardiography, patients were divided into PapMI with MR, PapMI (PapMI without MR), and non-PapMI groups. All patients underwent emergency coronary angiography and coronary intervention. The exclusion criteria were as follows: aged >85 years, renal insufficiency, cardiac shock, patients with contraindications for CMR, cardiomyopathy, and mitral valve disease history. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was taken from all the patients. The ethics committee of Drum Tower Hospital affiliated to Medical School of Nanjing University and Xinxiang Central Hospital approved the study protocol (No.2019-190-03). Bias control through multicenter study and reduce the rate of loss to follow-up. Study profile see Figure 1.
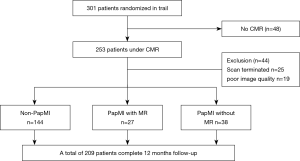
Intervention
Coronary angiography was performed by the radio or femoral approach. All patients underwent P-PCI and stenting according to standard techniques. Stent implantation was successfully completed in all patients.
CMR protocol and analysis
All imaging was performed on a 1.5 T Philips Achieva Cardiac MR scanner (Philips HealthCare, Best, NL, USA). Breath-hold LGE short-axis images were acquired ~15 minutes after injection of 0.2 mmol/kg Gd-DTPA (GE Pharmacy, Shanghai, China). Imaging parameters for the 2D LGE were: TR/TE, 6/3; FA, 25°; TI 260–350 ms; voxel, 1.6×1.9×8 mm3; and shot duration 100–125 ms. CMR was performed 4.8±1.9 days after PCI. The CMR images were analyzed by a cardiac radiologist blinded to patient history and clinical outcome, using commercially available software (Extended MR WorkSpace 2.6.3.5, Philips Medical Systems, Best, The Netherlands). The MR exam included assessment of cardiac function, flow, anatomy, and viability. Left ventricular (LV) ejection fraction (EF), mitral regurgitation (MR) fraction, and LV dimensions as measured by cardiac MR were obtained. The typical PapMI of images of CMR see Figure 2.
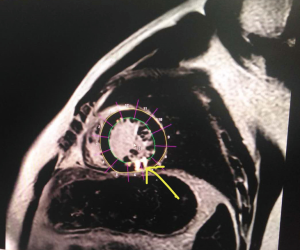
Echocardiography
Based on the standard clinical practice in accordance with the American Society of Echocardiography recommendations (9), transthoracic Doppler echocardiography was performed with a commercially available ultrasound machine (Vivid-7, General Electric, Horten, Norway). The severity of MR was scored as mild (regurgitant orifice area <0.2 cm2), moderate (regurgitant orifice area, 0.2 to 0.4 cm2), or severe (regurgitant orifice area >0.4 cm2). In this study, MR was defined as greater than moderate grade.
Cardiovascular events
The primary clinical endpoint was defined as a composite of cardiovascular death, reinfarction, in-stent restenosis (ISR), readmission, and heart failure within 12 months after infarction. A blinded independent clinical events committee adjudicated all potential cardiovascular events based on prospectively defined guidelines (10,11). All patients received follow-up in outpatient clinics, or by telephone and email. Follow-up was performed every 3 months.
Statistical analysis
Data were statistically evaluated using dedicated software (SPSS version 24.0 SPSS Inc., CA, USA). Comparisons of continuous variables were performed with 1-way ANOVA analysis. Categorical variables were compared with the Wilcoxon signed-rank test. The Chi-squared test was used to compare proportions. Binary logistic regression with stepwise backward selection was used to screen the independent variables, and odds ratio (OR) and confidence intervals (CI) were calculated. All statistical tests were 2-sided. P<0.05 indicated a statistically significant difference.
Results
Clinical characteristics of the study population
A total of 209 patients were enrolled in the current study. Clinical characteristics of the patients are presented in Table 1. A total of 27 patients with a median age of 66.8 years showed PapMI with MR after PCI (PapMI with MR group) and 38 patients with a median age of 61.2 years showed PapMI without MR after PCI (PapMI group), whereas 144 patients did not develop PapMI after PCI (non-PapMI group), with a median age of 57.0 years. Compared with the non-PapMI and PapMI group, the PapMI with MR group had more advanced age (P=0.001), higher troponin I (P<0.001), higher SYNTAX score (P<0.001), more diabetics (P=0.006), and longer time to reperfusion [symptom onset-to-balloon (SO to B), P=0.001] at presentation. There were no differences in clinical parameters including gender, BMI, smoking status, hypertension, medication history, and other baseline characteristics among the 3 groups.
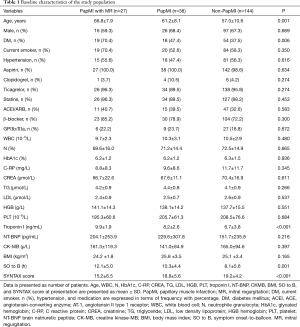
Full table
CMR characteristics of the study population
The CMR characteristics of the subjects are presented in Table 2. Compared with the non-PapMI and PapMI groups, the PapMI with MR group had more microvascular occlusions (MVO) (P=0.004) and impaired left ventricular ejection fraction (LVEF) (P=0.002). There were no differences in LV volume parameters, including infarct size, left ventricular end-systolic volume (LVSDV), left ventricular end-diastolic volume (LVEDV), and left atrium diameter (LAD) among the 3 groups.

Full table
Predictors of PapMI with MR
Binary logistic regression with stepwise backward selection analysis were implemented to evaluate the independent prognostic factors for the incidence of PapMI with MR. Significant predictors are shown in Table 3. The independent predictors of the presence of PapMI with MR were age [OR, 1.10 (CI, 1.03–1.17); P=0.003), presence of MVO [OR, 4.37 (CI, 1.19–16.0); P=0.026], and impaired LVEF [OR, 0.91 (CI, 0.86–0.97); P=0.004].

Full table
Clinical outcomes at 1-year follow-up
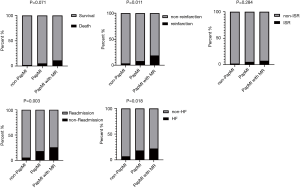
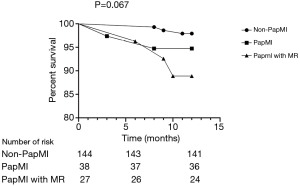
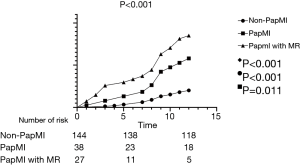
Clinical outcomes were assessed at the 1-year follow-up visit for all patients. At 1-year follow-up, there were no significant differences in death [non-PapMI, 3 (2.1%) vs. PapMI, 2 (5.3%) vs. PapMI with MR, 3 (11.1%); P=0.071] and ISR [non-PapMI, 3 (2.1%) vs. PapMI, 2 (5.3%) vs. PapMI with MR, 2 (7.4%); P=0.284] among the 3 groups (Table 4, Figures 3,4). There were significantly higher occurrences of nonfatal reinfarctions (P=0.011), readmission (P=0.003) and heart failure (P=0.018) in the PapMI with MR group (Table 4; Figure 3). Accordingly, major adverse cardiovascular events (MACE) at 1-year follow-up were significantly higher in the PapMI with MR group (P<0.001; Table 4, Figure 5).
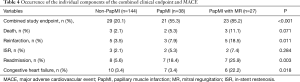
Full table
Discussion
It is well known that PapMI is associated with MR following myocardial infarction (1,12-16). To the best of our knowledge, however, no studies have shown the real-world prognostic significance of PapMI with MR in patients with STEMI diagnosed by CMR. In this study, we found that the incidence of PapMI with MR was 13%, indicating that the presence of PapMI with MR is not uncommon in the present day. Furthermore, the incidence of PapMI with MR was mainly related to advanced age, MVO detected by CMR, and impaired LV function. PapMI with MR had significantly more MACE compared with the PapMI and non-PapMI groups. However, mortality did not significantly increase at 1-year follow-up.
Compared to results published by Tanimoto et al. (1,12-16) reporting a prevalence of 40%, PapMI was observed more frequently in the present study with a prevalence of 45%. We detected 27 PapMI with MR cases among 209 STEMI patients (the incidence was 13%), and 65 PapMI patients (the incidence was 42%), suggesting that PapMI with MR is not so rare in STEMI and is common in PapMI. It’s similar to the result of Chinitz et al. (17).
Advanced age is a traditional risk factor for STEMI. Our study demonstrated that age [OR, 1.10 (CI, 1.03–1.17), P<0.001] was an independent predictor of PapMI with MR. This was also confirmed in a study by Bouma et al. (18).
MVO indicates a lack of adequate tissue perfusion within the infarcted myocardium, and is an independent risk factor for PapMI as visualized by CMR in previous studies. This conclusion is consistent with our study. PapMI was also related to obvious myocardial damage and MVO as detected by CMR. Others (19) have confirmed that PapMI was associated with severe reperfusion injury such as MVO and hemorrhage. However, our study is the first to use CMR to assess PapMI combined with MR including infarct extent and severe reperfusion injury such as MVO. Therefore, our results support previous findings by illustrating that MVO was an independent predictor for PapMI combined with MR [OR, 4.37 (CI, 1.19–16.0), P=0.028]. Grieve et al. (19). showed that impaired LV function was an independent risk factor for the presence of PapMI, and our study showed similar findings in the PapMI with MR group [OR, 0.91 (CI, 0.86–0.97), P=0.004].
Ischemic MR has been considered an independent risk factor of increased mortality in patients with PapMI (12,15,20-22). However, very few studies have reported on prognosis in PapMI with MR. Our study is the first to demonstrate that PapMI with MR has a poor prognosis. In our study, patients with PapMI with MR had significantly more MACE compared to the PapMI and non-PapMI groups [PapMI with MR, 23 (85.2%) vs. PapMI, 21 (55.3%) vs. non-PapMI, 29 (20.1%)] at 1-year follow-up (P<0.001, respectively). However, similar to previous results (23), there were no significant differences in mortality rates among the 3 groups (P=0.071).
In view of the relationship between PapMI and MR, we should pay attention to patients have suffered PapMI combined with MR, and beware patients with MR diagnosed by echocardiography caused by PapMI.
Conclusions
The incidence of PapMI with MR as determined by CMR imaging is not uncommon in STEMI patients after pPCI. Compared to the PapMI and non-PapMI groups, the PapMI with MR group had worse prognosis. Our data contributes to an improved understanding of the clinical characteristics of PapMI with MR, and may help aid in the prevention of MACE.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-3476
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-3476
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-3476). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was taken from all the patients. The ethics committee of Drum Tower Hospital affiliated to Medical School of Nanjing University and Xinxiang Central Hospital approved the study protocol (No.2019-190-03).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tanimoto T, Imanishi T, Kitabata H, et al. Prevalence and clinical significance of papillary muscle infarction detected by late gadolinium-enhanced magnetic resonance imaging in patients with ST-segment elevation myocardial infarction. Circulation 2010;122:2281-7. [Crossref] [PubMed]
- Eitel I, Gehmlich D, Amer O, et al. Prognostic relevance of papillary muscle infarction in reperfused infarction as visualized by cardiovascular magnetic resonance. Circ Cardiovasc Imaging 2013;6:890-8. [Crossref] [PubMed]
- Nappi F, Nenna A, Sing SSA, et al. Mitral regurgitation: lessons learned from COAPT and MITRA-Fr. J Thorac Dis 2020;12:2936-44. [Crossref] [PubMed]
- Simonetti OP, Kim RJ, Fieno DS, et al. An improved MR imaging technique for the visualization of myocardial infarction. Radiology 2001;218:215-23. [Crossref] [PubMed]
- Pitt B, Fonarow GC, Gheorghiade M, et al. Improving outcomes in post-acute myocardial infarction heart failure: incorporation of aldosterone blockade into combination therapy to optimize neurohormonal blockade. Am J Cardiol 2006;97:26F-33F. [Crossref] [PubMed]
- Kim RJ, Fieno DS, Parrish TB, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 1999;100:1992-2002. [Crossref] [PubMed]
- Amado LC, Gerber BL, Gupta SN, et al. Accurate and objective infarct sizing by contrast-enhanced magnetic resonance imaging in a canine myocardial infarction model. J Am Coll Cardiol 2004;44:2383-9. [Crossref] [PubMed]
- Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003;16:777-802. [Crossref] [PubMed]
- Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med 2015;372:1291-300. [Crossref] [PubMed]
- Douglas PS, Hoffmann U, Lee KL, et al. PROspective Multicenter Imaging Study for Evaluation of chest pain: rationale and design of the PROMISE trial. Am Heart J 2014;167:796-803.e1. [Crossref] [PubMed]
- Weiss SR, Coppola J, Gambino J, et al. Isolated acute papillary muscle infarction in the absence of coronary artery disease resulting in cardiogenic shock and emergent mitral valve replacement. Cathet Cardiovasc Diagn 1998;43:185-9. [Crossref] [PubMed]
- Bax JJ, Delgado V. Papillary muscle infarction, mitral regurgitation, and long-term prognosis. Circ Cardiovasc Imaging 2013;6:855-7. [Crossref] [PubMed]
- Curtis E, Corkill M, Amir N, et al. Acute papillary muscle infarction and rupture in the puerperium complicating Libman-Sacks endocarditis in a patient with systemic lupus erythematosus and antiphospholipid syndrome: a case report. Eur Heart J Case Rep 2019;3:1-4. [Crossref] [PubMed]
- van Mourik MJ, Zaar DV, Smulders MW, et al. Adding Speckle-Tracking Echocardiography to Visual Assessment of Systolic Wall Motion Abnormalities Improves the Detection of Myocardial Infarction. J Am Soc Echocardiogr 2019;32:65-73. [Crossref] [PubMed]
- Klug G, Feistritzer HJ, Reinstadler SJ, et al. Impact of posteromedial papillary muscle infarction on mitral regurgitation during ST-segment elevation myocardial infarction. Int J Cardiovasc Imaging 2020;36:503-11. [Crossref] [PubMed]
- Bretschneider C, Heinrich HK, Seeger A, et al. Impact of Papillary Muscle Infarction on Ischemic Mitral Regurgitation Assessed by Magnetic Resonance Imaging. Rofo 2018;190:42-50. [Crossref] [PubMed]
- Chinitz JS, Chen D, Goyal P, et al. Mitral apparatus assessment by delayed enhancement CMR: relative impact of infarct distribution on mitral regurgitation. JACC Cardiovasc Imaging 2013;6:220-34. [Crossref] [PubMed]
- Bouma W, Willemsen HM, Lexis CP, et al. Chronic ischemic mitral regurgitation and papillary muscle infarction detected by late gadolinium-enhanced cardiac magnetic resonance imaging in patients with ST-segment elevation myocardial infarction. Clin Res Cardiol 2016;105:981-91. [Crossref] [PubMed]
- Grieve SM, Bhindi R, Seow J, et al. Microvascular obstruction by intracoronary delivery of mesenchymal stem cells and quantification of resulting myocardial infarction by cardiac magnetic resonance. Circ Heart Fail 2010;3:e5-6. [Crossref] [PubMed]
- Aldrovandi A, De Ridder SP, Strohm O, et al. Detection of papillary muscle infarction by late gadolinium enhancement: incremental value of short-inversion time vs. standard imaging. Eur Heart J Cardiovasc Imaging 2013;14:495-9. [Crossref] [PubMed]
- Madu EC, D'Cruz IA. The vital role of papillary muscles in mitral and ventricular function: echocardiographic insights. Clin Cardiol 1997;20:93-8. [Crossref] [PubMed]
- Bizzarri F, Mattia C, Ricci M, et al. Cardiogenic shock as a complication of acute mitral valve regurgitation following posteromedial papillary muscle infarction in the absence of coronary artery disease. J Cardiothorac Surg 2008;3:61. [Crossref] [PubMed]
- Grigioni F, Enriquez-Sarano M, Zehr KJ, et al. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation 2001;103:1759-64. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)


