
Mechanical properties of de-epithelialized tracheal allografts
Introduction
Tracheal defect is a common, life-threatening, clinical disease that is mainly caused by tumor, trauma, long-time intubation, or congenital disease. Tracheal defects within 6 cm in size can be repaired by anastomosis, while tracheal defects larger than 6 cm are difficult to repair. Tracheal transplantation is considered a relatively ideal method to repair large area tracheal defects. Clinical cases of successful allograft tracheal transplantation have been reported (1). However, immunosuppressive agents are required after transplantation, which increase the probability of tumor recurrence and opportunistic infection. The trachea has very low antigenicity compared with other organs (2,3). Furthermore, its antigenicity has been found to be mainly located in the mucous layer of the trachea, with only slight immunogenicity being present in the cartilage (4). Antigenicity of tracheal grafts can be significantly reduced by removing epithelial cells and mixed glands (5). Research indicates that there is no need to use immunosuppressive agents in allogeneic tracheal transplantation performed through de-epithelialization (5,6). Another requirement for successful tracheal transplantation is the maintenance of patency; thus, maintaining the supporting role of tracheal cartilage must be considered while removing immunogenicity. There are many methods (physical, chemical, or enzymatic) used for de-epithelialization, and different methods can mechanically affect tissue in several ways. For example, the sodium dodecyl sulfate (SDS), Triton X-100, and enzymatic agents all have different effects on the mechanical properties of the porcine aortic valve (7). However, Triton X-100 only exerts a mild effect and cannot effectively remove cell components after a short period of treatment (8,9). Meanwhile, SDS is powerful decellularizing agent, which can rapidly remove cellular components (10). Tracheal reconstruction should be performed promptly for patients with diseases such as cancer or traumatic injury. Therefore, a simple and fast method of decellularization is needed to reduce the wait time for treatment. SDS may be an ideal candidate for this, by virtue of its decellularization power and its ability to tighten collagen (11) and generate a more fibrous substrate (12); however, it is not clear whether or not the application of SDS will affect the mechanical properties of the trachea. The purpose of this study was thus to determine whether or not the tensile and compressive mechanical properties of trachea are altered after SDS de-epithelialization and allograft implantation. We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-2739).
Methods
Trachea collection
This study was conducted at the Center for Laryngotracheal Reconstruction at Tangdu Hospital, affiliated with the Air Force Military Medical University. The study was approved by the Laboratory Animal Welfare and Ethics Committee of the Air Force Medical University (IACUC-20191009), and conducted in compliance with Air Force Medical University guidelines for the care and use of animals. The donor mixed-breed dogs were anesthetized with an intramuscular injection of xylazine hydrochloride (2 mg/kg) and diazepam (0.4 mg/kg). After anesthesia, the dogs were laid on the operating table, the hair of the middle neck was removed, the skin was incised, and the anterior cervical muscles were separated to expose the cervical trachea. The cervical tracheas of 20 mongrel dogs (~10 cm in length) were obtained, and then all animals were euthanized. The surrounding connective tissues were removed and divided into 4 groups with 5 samples in each group. Group A (the control group), consisted of fresh canine tracheas; group B, consisted of tracheas de-epithelialized with SDS; group C, consisted of tracheas de-epithelialized with SDS and then heterotopically implanted (into the latissimus dorsi of beagle dogs) for 1 month; group D, consisted of tracheas de-epithelialized with SDS and then heterotopically implanted for 6 months. Between collection and testing, the 4 groups of collected tracheas were immersed in phosphate buffered saline (PBS) to prevent drying. All mechanical tests were completed within 6 hours of collection.
De-epithelialized tracheas
First, 5 g SDS powder was dissolved in 500 mL deionized water to create 1% SDS solution, and then a 0.22-µm fiber membrane was used to filter and sterilize the solution. With the exception of group A (the control group), all tracheas were first de-epithelialized with 1% SDS in the fallowing fashion (10): The proximal end of the trachea was blocked with a silicone plug. The tracheas were placed in test tubes containing SDS solution on the luminal side and hypertonic citrate adenine solution (Shanghai Blood Center, Shanghai, China) on the exterior side. Then, all tubes were placed in a 4 °C refrigerator for 72 hours for de-epithelialization, and the SDS solution was changed every day into the incubation. The tracheas in group B (n=5) were removed and immersed in PBS, and the compressive and tensile tests were performed within 6 hours of removal. One cartilage ring per trachea was taken before the test for hematoxylin and eosin (HE) staining. The remaining 10 tracheas, which were those of groups C and D, were heterotopically implanted.
Allografting of de-epithelialized tracheas
After de-epithelialization, the tracheas of group C and group D were disinfected with 0.5% iodophor and repeatedly rinsed with approximately 2,000 mL of normal saline. The cartilage rings at both ends were then removed and the tracheas were cut to a length of approximately 6 cm, and a 1–1.5 cm incision was made between 2 of the rings in each trachea. Each processed trachea was fixed to a silicone tube of the same length; the tubes had small holes in their walls, and their ends were sutured together with absorbable threads. This treatment was performed to prevent the tracheas from becoming deformed under pressure after implantation.
We selected 10 healthy adult beagles weighing about 12–15 kg to be randomly assigned as ectopic transplanted animals, and each trachea was implanted on a beagle’s back. The specific operation procedure was as follows: an L-shaped incision was made in the latissimus dorsi of a beagle; the skin was cut, the fascia layer was exposed, and the treated trachea was embedded into the fascia layer and sutured in place. All the animals were housed and fed by a specialist breeder in the Animal Feeding Center. The group C tracheas were removed 1 month after implantation, and the group D tracheas were removed 6 months after implantation. The excised tracheas were immediately immersed in PBS, and compressive and tensile testing were performed within 6 hours. Before the tests, a ring was taken from the end of each trachea for HE staining.
Tracheal histology
Before mechanically testing each group of tracheas, we took a ring from one end of each trachea for routine preparation and histological examination. The tracheal rings were placed in 10% neutral formalin buffer and fixed at room temperature for 24 hours. After fixation, the rings were washed with distilled water, dehydrated with a graded ethanol series, embedded in paraffin, and sliced at a thickness of 4 µm. The sections were stained with HE.
Mechanical testing equipment
All biomechanical tests were completed at the State Key Laboratory of Mechanical Structural Strength and Vibration, Xi’an Jiaotong University. The tests were performed on a single-column benchtop universal testing machine (UTM) (model 5848, Instron, Norwood, MA, USA) equipped with 2 kN load cells. All experimental tests were carried out at room temperature and 50% humidity. Software (Bluehill, version 5.53.00, Instron) was used to control test execution and data collection. The load (N) and displacement (%) data of each experiment were obtained and exported to a spreadsheet program (Excel, version 14.2.5, Microsoft, Redmond, WA, USA). The same instrument was used for all biomechanical tests.
Compressive strength test
The specimens that were used for the compressive strength test (n=5) contained 3 tracheal rings each. Electronic digital display calipers (LINKS 0–150 mm, HMCT Group, Harbin, China) were used to measure the initial anterior-posterior diameter of the trachea. All mechanical testing procedures were completed within 6 hours of tissue collection. Each specimen was placed between 2 smooth, parallel, disc-shaped plates to distribute the load evenly along the trachea. The 2 discs were fixed on the UTM load arm (Figure 1A), and the specimen was compressed at a speed of 5 mm/min. When the anterior-posterior diameter was reduced to 50% of the initial diameter, the measurement was stopped (Figure 1B). The compression radial load (N) of each tracheal section at 50% displacement was calculated using a spreadsheet.
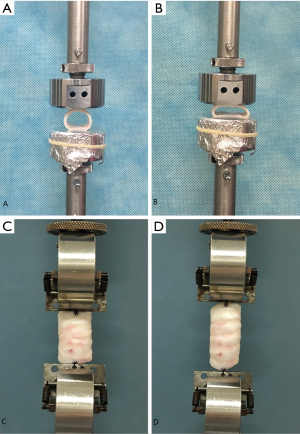
Tensile strength test
Each specimen for the tensile strength test was composed of 6 tracheal rings (n=5), and all were subjected to an axial elongation test. A fixture was placed in parallel with the load cell and was used to actively pull the specimen. The other end, which was at the bottom, pulled the other end of the specimen. The outermost membrane regions on both ends of the trachea were sutured to the fixture with a 4-0 Mersilk suture (braided silk, Ethicon, Somerville, NJ, USA) (Figure 1C). The original length before stretching was measured using electronic digital display calipers (LINKS 0–150 mm, HMCT Group, Harbin, China), and the specimen was stretched at a speed of 5 mm/min. When the trachea was stretched to 150% of the original length (50% displacement), the measurement was stopped (Figure 1D). The tensile load (N) of each tracheal specimen at 50% displacement was calculated using a spreadsheet.
Statistical analysis
All statistical analyses were performed using SAS software (Version 9.3, SAS Institute Inc., Cary, NC, USA), with an α of 0.10 as the threshold for statistical significance. One-way analysis of variance (ANOVA) was used to test the differences in compression and tension results among groups. The Tukey-Kramer multiple comparison method was used to examine the pairwise differences between specific groups in each mechanical test.
Results
Macroscopic evaluation of allografts
After 1 and 6 months of implantation, the tracheal surface was covered with a thin layer of fascia, and abundant capillaries could be seen on the surface. With the extension of implantation time, small columnar granulation tissue could be seen growing in the lumen center, but there was no obvious stenosis in the lumen.
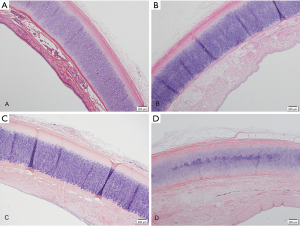
Results of histological examination of the trachea
The fresh canine tracheas had a pseudostratified columnar epithelium visible under the microscope (Figure 2A). After de-epithelialization, the tracheal epithelium was almost absent, leaving only the extracellular matrix, and the chondrocytes were still alive (Figure 2B). On microscopic examination at 1 and 6 months post-implantation, the epithelial layer was filled with fibroblasts (Figure 2C,D).
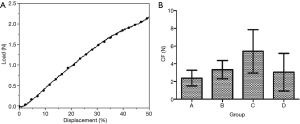
Compressive strength test results
The compression curves of each group conformed to the typical compression displacement curve (Figure 3A). The mean compressive strength of each group is presented in Figure 3B. There was no significant difference in compressive strength among the groups (Table 1). During the test, the tracheas returned to its original state after the pressure was removed.

Full table
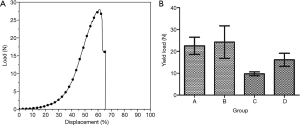
Tensile strength test results
The representative load-displacement curve of tracheas during elongation testing, in which rupture occurred at 61% of displacement, is shown in Figure 4A. The rupture point of all specimens happened after 50% elongation and each load-displacement curve showed an obvious deviation after 50% of displacement. The shape of the tensile load-displacement curve was similar with one trachea to the next; therefore, in this experiment, we used the load at 50% tensile displacement to calculate the tensile strength values that we compared among groups. The mean tensile strength was calculated for each group (Figure 4B). We found that tensile strength was significantly reduced in group C compared to the other 3 groups, whereas there was no significant difference among groups A, B, and D (Table 1).
Discussion
Tracheal defects less than 6 cm can be repaired by resection and end-to-end anastomosis. However, long-segment lesions require tracheal replacement (13), which is difficult to perform. Transplantation is the currently used and more consistent repair method is. Liu et al. (5) performed heterotopic transplantation of tracheas from dogs in which the tracheal mucosa was treated with a detergent (1% Triton X-100 t-octylphenoxypolyethoxyethanol; T-9284) without using any immunosuppressants, and 5 of the 6 grafts were confirmed to have been successfully transplanted. Hung et al. (14) also reported significant airway epithelial regeneration on the surface of the rabbit tracheal lumen after reconstruction with an acellular stent, but the tracheal stent eventually collapsed, leading to death. Therefore, ensuring the mechanical properties of the trachea and the lack of airway obstruction is one of the main obstacles that must be overcome in tracheal reconstruction. For the de-epithelialized trachea to withstand the intrachamber airway pressure fluctuations associated with breathing, the elasticity and lateral strength of the trachea must be very close to those of the natural trachea (15,16). At present, there is no consensus on the biomechanical properties of the trachea in animal models (17-21). Therefore, we used fresh dog tracheas as a control to determine whether there were any changes in the mechanical properties of the de-epithelialized trachea 1 and 6 months after allografting and when, if at all, these changes occurred. We determined whether the biomechanical properties of the dog tracheas were greatly affected when allografts were successfully completed. We also tried to determine whether de-epithelialized trachea could be applied for allografting.
The compressive load-displacement curve of the canine trachea was a typical compression test curve (22). With increasing displacement, the load also increases exponentially. When the external pressure was removed, all samples returned to their original shape, indicating that the tracheas of each group were highly elastic. The average strength of the canine trachea under 50% radial compression in each group confirmed that the tracheas of all groups had good radial support capacity and elasticity. There was no statistically significant difference in compression load between the groups.
The tensile curve in this experiment was similar to the load-displacement curve of biological tissue subjected to tensile loading (22). The curve started with a steady and gradual rise, followed by a linear rise after 20–35% displacement, and a sharp inflection point at the time of tissue rupture. In this experiment, the fracture point appeared in all the samples after 50% elongation, and the load-displacement curve deviated greatly after this point. No fracture was found in any of the samples before 50% displacement, and the curves of the samples were consistent. Therefore, in this experiment, we adopted the load value at 50% displacement for the intergroup comparisons.
The tensile load had decreased as of 1 month after heterotopic implantation, and there was a significant difference compared with the fresh dog tracheas. The difference may be attributable to the inter-ring incisions made before implantation (the purpose of the incisions was to provide a better nutrient supply to the implanted trachea from the fascia to improve its survival), which reduced the connection between rings, making the trachea easier to pull apart. At 6 months after allografting, the tensile loading capacity had increased. This increase may be related to the interannular parenchyma and the occurrence of gradual fibrosis, which could affect the tensile strength of the trachea and increase the maximum tensile load. There was no statistically significant difference among the newly de-epithelialized group, the fresh dog trachea group, and the 6-month allograft group. Moreover, our results were similar to the biomechanical values measured by Lee et al. (23).
The biomechanical properties of the trachea arise mainly from the tracheal cartilage rather than the mucosa (24). This is also consistent with the fact that the scaffolding effect of the cartilage was not impacted, since only the epithelial cells of the tracheal mucosa were removed during the process of de-epithelialization.
The present experiments had certain limitations. It would have been ideal to test and retest the same tracheas from the same mixed-breed dogs, from collection to de-epithelialization to 1 and 6 months after implantation, as this would have controlled for the individual differences between the different mixed-breed dogs’ tracheas and increased the precision of the data (due to individual differences between the dogs, some tracheas were thicker and had harder cartilage, while other tracheas were thinner and had softer cartilage). However, the tracheas we implanted were all approximately 6 cm in length, and due to the limited length of the cervical trachea, it was difficult to reduce the presence of individual differences.
Conclusions
Our data suggest that during the entire treatment, the compression strength of the trachea was not affected. An interloop incision was made between the trachea rings before performing the allograft; the tensile force of the trachea after performing the allograft was affected for a short time, but was not affected 6 months after the procedure. Moreover, the tracheal cartilage of dogs was not rejected after 1% SDS de-epithelialization and observation of long-term implantation for 6 months, and the mechanical properties of the trachea were not significantly changed compared with fresh trachea. This provides the basic data for the repair of long-segment tracheal defects without immunosuppression and lays the experimental foundation for further orthotopic tracheal transplantation and re-epithelialization.
Acknowledgments
This work was carried out by Centre for Laryngotracheal Reconstruction at Tangdu Hospital, affiliated with the Air Force Military Medical University. We gratefully acknowledge to Shaanxi Province Research Center of Cell Immunological Engineering and Technology for their generous assistance, as well as to Yangmeng Feng and Huimin Li for their invaluable cooperation of in the research process.
Funding: This study was supported by the Natural Science Basic Research Plan of Shaanxi Province of China (No. 2018JM7145), and the Innovation Foundation of Tangdu Hospital (No. 2012LCYJ001).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-2739
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-2739
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-20-2739
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-2739). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Laboratory Animal Welfare and Ethics Committee of the Air Force Medical University (IACUC-20191009), and conducted in compliance with Air Force Medical University guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Delaere P, Vrancks J, Verleden G, et al. Tracheal allotransplantation after withdrawal of immunosuppressive therapy. N Engl J Med 2010;362:138-45. [Crossref] [PubMed]
- Rose KG, Sesterhenn K, Wustrow F. Tracheal allotransplantation in man. Lancet 1979;1:433. [Crossref] [PubMed]
- Lane BP, Habicht GS, Jasper GS. Lymphocyte-epithelium interaction during rejection of nonisogeneic rat tracheal grafts. Am J Pathol 1977;86:71-80. [PubMed]
- Beigel A, Steffens-Knutzen R, Müller B, et al. Tracheal transplantation. III. Demonstration of transplantation antigens on the tracheal mucosa of inbred rat strains. Arch Otorhinolaryngol 1984;241:1-8. [Crossref] [PubMed]
- Liu Y, Nakamura T, Yamamoto Y, et al. Immunosuppressant-free allotransplantation of the trachea: the antigenicity of tracheal grafts can be reduced by removing the epithelium and mixed glands from the graft by detergent treatment. J Thorac Cardiovasc Surg 2000;120:108-14. [Crossref] [PubMed]
- Jacobs JP, Elliott MJ, Haw MP, et al. Pediatric tracheal homograft reconstruction: a novel approach to complex tracheal stenoses in children. J Thorac Cardiovasc Surg 1996;112:1549-58. [Crossref] [PubMed]
- Liao J, Joyce EM, Sacks MS. Effects of decellularization on the mechanical and structural properties of the porcine aortic valve leaflet. Biomaterials 2008;29:1065-74. [Crossref] [PubMed]
- Remlinger NT, Czajka CA, Juhas ME, et al. Hydrated xenogeneic decellularized tracheal matrix as a scaffold for tracheal reconstruction. Biomaterials 2010;31:3520-6. [Crossref] [PubMed]
- Elder BD, Eleswarapu SV, Athanasiou KA. Extraction techniques for the decellularization of tissue engineered articular cartilage constructs. Biomaterials 2009;30:3749-56. [Crossref] [PubMed]
- Ott HC, Matthiesen TS, Goh SK, et al. Perfusion-decellularized Matrix: Using Nature's Platform to Engineer a Bioartificial Heart. Nat Med 2008;14:213-21. [Crossref] [PubMed]
- Zhou J, Fritze O, Schleicher M, et al. Impact of heart valve decellularization on 3-D ultrastructure, immunogenicity and thrombogenicity. Biomaterials 2010;31:2549-54. [Crossref] [PubMed]
- O'Neill JD, Anfang R, Anandappa A, et al. Decellularization of human and porcine lung tissues for pulmonary tissue engineering. Ann Thorac Surg 2013;96:1046-55. [Crossref] [PubMed]
- Den Hondt M, Vranckx JJ. Reconstruction of defects of the trachea. J Mater Sci Mater Med 2017;28:24. [Crossref] [PubMed]
- Hung SH, Su CH, Lin SE, et al. Preliminary experiences in trachea scaffold tissue engineering with segmental organ decellularization. Laryngoscope 2016;126:2520-7. [Crossref] [PubMed]
- Grillo HC. Tracheal replacement: a critical review. Ann Thorac Surg 2002;73:1995-2004. [Crossref] [PubMed]
- Belsey R. Resection and reconstruction of the intrathoracic trachea. Br J Surg 1950;38:200-5. [Crossref] [PubMed]
- Hashem FK, Al Homsi M, Mahasin ZZ, et al. Laryngotracheoplasty using the Medpor implant: an animal model. J Otolaryngol 2001;30:334-9. [Crossref] [PubMed]
- Carron JD, Greinwald JH, Oberman JP, et al. Simulated reflux and laryngotracheal reconstruction: a rabbit model. Arch Otolaryngol Head Neck Surg 2001;127:576-80. [Crossref] [PubMed]
- Coppit G, Perkins J, Munaretto J, et al. The effects of mitomycin-C and stenting on airway wound healing after laryngotracheal reconstruction in a pigmodel. Int J Pediatr Otorhinolaryngol 2000;53:125-35. [Crossref] [PubMed]
- Kojima K, Bonassar LJ, Roy AK, et al. Autologous tissue engineered trachea with sheep nasal chondrocytes. J Thorac Cardiovasc Surg 2002;123:1177-84. [Crossref] [PubMed]
- Hamaide A, Arnoczky SP, Ciarelli MJ, et al. Effects of age and location on the biomechanical and biochemical properties of canine tracheal ring cartilage in dogs. Am J Vet Res 1998;59:18-22. [PubMed]
- Fung YC. Biomechanics: Mechanical Properties of Living Tissues. 2nd edition. New York, NY: Springer-Verlag, Inc., 1993.
- Lee JS, Park J, Shin DA, et al. Characterization of the biomechanical properties of canine trachea using a customized 3D-printed apparatus. Auris Nasus Larynx 2019;46:407-16. [Crossref] [PubMed]
- Wang JY, Mesquida P, Lee T. Young's modulus measurement on pig trachea and bronchial airways. Annu Int Conf IEEE Eng Med Biol Soc 2011;2011:2089-92. [PubMed]
(English Language Editors: J. Jones and J. Gray)


