
Prognostic significance of radiodensity-based skeletal muscle quantification using preoperative CT in resected non-small cell lung cancer
Introduction
Depletion of skeletal muscle mass, refers to sarcopenia, is an important health implication in old age because the status is associated with injuries, frailty, and mortality. As an important component of cancer cachexia syndrome, the presence of sarcopenia is also being increasingly recognized in oncologic patients (1,2), when it present, the patients are more likely to have poor functional status and shorter overall survival (3,4).
An original article entitled “Preoperative Computed Tomography-Determined Sarcopenia and Postoperative Outcome after Surgery for Non-Small Cell Lung Cancer” was recently published in the Scandinavian Journal of Surgery (5). In the study, skeletal muscle area at the level of the third lumbar (L3) vertebra was quantified using initial CT images at the time of lung cancer diagnosis using the conventional methods. Specifically, skeletal muscle mass at L3 level was quantified using −29 to 150 HU, which was corresponding HU for skeletal muscle. Sarcopenia was considered when the L3 skeletal muscle index was <55 cm2/m2 in men and <39 cm2/m2 in women, as proposed by the international consensus group for cancer cachexia (1). Based on the previous study (5), it may be concluded that the presence of preoperative sarcopenia does not appear to have a negative impact on patient-related outcomes following resection of NSCLC. However, the presence of sarcopenia was found to be an independent poor prognostic predictor in the meta-analysis (6), although the methods and cut-off for the quantification of skeletal muscle mass are slight different between studies.
Our research group developed an automatic segmentation method for skeletal muscle using machine learning algorithm, which also enables subsequent quantification of the skeletal muscle based on radiodensity level as low attenuation muscle (−29 to <30 HU) and high attenuation muscle (30 to 150 HU). In the present study, we aimed to demonstrate the prognostic implications of preoperative CT-based detailed quantification of the skeletal muscle using different radiodensity levels for the same cohort of resected lung cancer patients with further clinical follow-up data.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-2344).
Methods
Patients
We retrospectively identified 272 consecutive patients with newly diagnosed, pathologically proven NSCLC who underwent curative-intent operation at Gachon University Gil Medical Center (GUGMC, Incheon, Korea) between January 2011 and December 2016. There were 164 men (60.3%) and the mean age was 62.9±9.6 years (range, 33–81 years). Body mass index (BMI) was defined as weight divided by height squared (kg/m2).
Perioperative and operative procedures
Patients were staged according to the American Joint Commission on Cancer (AJCC) Staging Manual (7th edition) (7). The routine preoperative workup included pulmonary function testing (PFT), bronchoscopy, chest computed tomography (CT), whole-body 18F-fluorodeoxyglucose (FDG) positron emission tomography/CT (PET/CT), and brain magnetic resonance imaging (MRI).
Eighteen (6.6%) patients received neoadjuvant chemotherapy. Operative procedures performed were as follows: lobectomy (n=238, 87.5%), bi-lobectomy (n=17, 6.3%), pneumonectomy (n=14, 5.1%), and segmentectomy (n=3, 1.1%). One hundred and fifty-one (55.5%) patients underwent minimal invasive surgery using the video assisted thoracoscopic surgery (VATS) technique.
PET/CT scan
All patients fasted for at least six hours to ensure normal blood glucose level. About one hour after administration of 370 MBq (10 mCi, i.v.) of FDG, PET imaging was performed (Siemens Medical Systems, Erlangen, Germany). The integrated CT imaging was performed from the head to the pelvic floor without administration of contrast using the following scan parameters: six-slice CT detectors, 130 kVp, 110 mAs, 2-mm pitch, 1-second tube rotation, and a slice thickness of 5 mm.
Image analyses
Skeletal muscle area was quantified using an in-house software (Gachon_DeepBody developed in the GUGMC, Incheon, Korea). For the Gachon_DeepBody, the U-Net architecture was used (8). For the hyper-parameters, the batch size was set to 8, learning rate was 0.0001, optimizer algorithm was Adam, and number of epochs was 150. The Gachon_DeepBody extracts muscle, subcutaneous fat, and visceral fat using the trained deep learning model, and measures the area for each body composition. Using the software, skeletal muscles were automatically identified and the area was calculated (Hounsfield unit: from −25 to 150 for skeletal muscle) on CT images. Moreover, the skeletal muscle could be classified based on radiodensity as very low attenuation muscle (−30 to 0 HU), low attenuation muscle (0 to 30 HU), and high attenuation muscle (30 to 150 HU) (Figure 1).
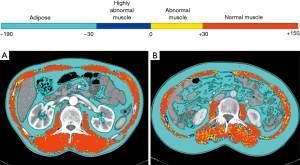
L3 skeletal muscle index (L3MI, cm2/m2) was defined as single cross-sectional area of total skeletal muscle at the L3 level, normalized for stature, as is conventional for BMI.
On this conventional method, the skeletal muscle mass has wide range of HU of −29 to 150. Sarcopenia was considered when the L3MI was <55 cm2/m2 in men and <39 cm2/m2 in women, as proposed by the international consensus group for cancer cachexia (1). However, because lower HUs represent more intramuscular fatty component, we subsequently assessed the proportion of low attenuation muscle (−29 to <30 HU) within the total skeletal muscle area (−29 to 150 HU). Using an optimal cut-off for the skeletal muscle density proportion (proportion of low attenuation muscle within the total skeletal muscle) determined by maximal chi-squared method, healthy/unhealthy muscle was defined.
Statistical analyses
Descriptive statistics have been reported as proportions (%) or means with standard deviations (SD). For categorical variables, comparisons between groups were performed using the Pearson’s χ2 test or Fisher’s exact test. Continuous variables were compared using the Student’s t-test.
Due to lack of consensus regarding the definition of healthy or unhealthy muscle (according to the proportion of low attenuation muscle within the total skeletal muscle), MaxStat, a maximal chi-squared method in open-source statistical software r (R Development Core Team, Vienna, Austria, http://www.R-project.org) was used to determine the optimal cut-off point.
Survival was estimated using the Kaplan–Meier method and compared using the log-rank test. Disease free survival (DFS) and overall survival (OS) were estimated from the time of surgery to recurrence, death, or last follow-up. DFS was calculated from the date of operation to the date of any recurrence (locoregional or distant) or the date of death from any cause. Univariable and multivariable Cox proportional hazard models were used to identify prognostic factors. Clinically important variables were included in the multivariable analysis, which was performed using the Enter method. Two-sided P values of <0.05 were considered statistically significant. The analyses were performed using SPSS for Windows ver. 19.0 (SPSS Inc., Chicago, IL, USA).
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The institutional review board of GUGMC approved this retrospective study and waived the requirement for informed patient consent (approval number: GCIRB 2020-251).
Results
Patient characteristics and radiodensity-based skeletal muscle mass
The overall prevalence of sarcopenia was 22.4% (32.9% in male and 6.5% in female). Presence of sarcopenia was significantly related to male gender (P<0.001) and smoking history (current or ex-smoker vs. non-smoker, P=0.003). Sarcopenic patients had a lower BMI (21.3±2.7 vs. 25.1±3.1 kg/m2, P<0.001) and a lower percentage forced expiratory volume in 1 second (FEV1) (87.2%±18.2% vs. 95.8%±19.5%, P=0.002).
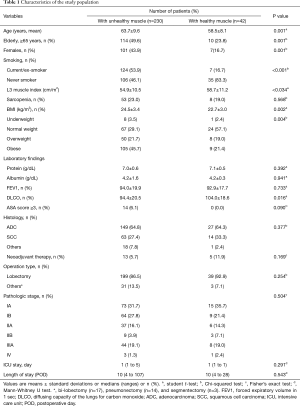
Using a maximal chi-squared method determined optimal cut-off for healthy or unhealthy muscle (proportion of low attenuation muscle, 24.5%), the prevalence of patient with healthy muscle was 15.4% (21.3% for male and 6.5% for female). The baseline characteristics of the patients with and without healthy muscle are presented in Table 1. The presence of healthy muscle was significantly related to male sex (P<0.001) and never smoker (83.3% vs. 46.1%, P<0.001). Patients with healthy muscle were younger (mean age, 58.5±8.1 vs. 63.7±9, P<0.001) and had a higher L3MI (58.7±11.2 vs. 54.9±10.5 cm/m2, P<0.034), lower BMI (22.7±3.0 vs. 24.5±3.4 kg/m2, P=0.002) and a higher DLCO (104.0%±18.6% vs. 94.4%±20.5%, P=0.016). However, no significant difference was observed in terms of preoperative protein and albumin levels, and percentage forced expiratory volume in 1 second (FEV1), tumor histology, or pathologic tumor stage.
Full table
Recurrence and survival outcomes
During the median follow-up of 37.9 months (range, 1.0–94.7 months), 87 patients (32.0%) experienced recurrence and 39 (14.3%) died.
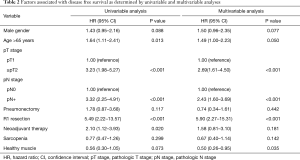
Between patients with and without sarcopenia, there was no significant difference on 3-year DFS rate (63.1% vs. 68.3%, P=0.481) or on 3-year OS rate (84.8% vs. 87.9%, P=0.576). However, patients with healthy muscle tended to show longer 3-year DFS rate (74.5% vs. 62.8%, P=0.131) and 3-year OS rate (92.6% vs. 86.1%, P=0.176), although statistically insignificant (Figure 2). In the multivariable analysis, the presence of healthy muscle (HR, 0.50, P=0.035) was one of the independent factors to predict 3-year DFS rate, along with R1 resection (HR, 5.90, P<0.001), pathologic T stage (HR, 2.69, P<0.001), and pathologic N stage (HR, 2.43, P<0.001) (Table 2).

Full table
Discussion
As an important indicator of cancer cachexia syndrome, the presence of sarcopenia has been shown to be a poor prognosticator, as the depletion of skeletal muscle mass is associated with injury, increased risk of chemotherapy-related toxicities, and reduced survival (1,4,9-11). However, the prevalence of sarcopenia varies based on the tumor type and stage, and the diagnostic methods to determine the condition. The pooled prevalence of sarcopenia was reported as 43% in NSCLC patients and 52% in small cell lung cancer patients (12). In meta-analysis, about 35% of NSCLC patients who surgically treated were sarcopenic and the presence of sarcopenia was also found to be an independent poor prognostic predictor (6). In thus, all six cohort studies determined sarcopenia by measuring the cross-sectional area of total skeletal muscle (four studies) or total psoas muscle (two studies) at the L3 level using preoperative CT images, and the cut-off values to determine sarcopenia differed between the studies. Furthermore, the prevalence of sarcopenia showed a wide variation from 14.0% to 55.6%.
In our study, no association was observed between the presence of preoperative sarcopenia and post-operative prognosis. This inconsistency may be due to the different cutoff value and included muscle amount for cross-sectional area to determine sarcopenia, and insufficient sample size with a relatively short-term follow-up period (median follow-up, 37.9 months). However, in the detailed radiodensity-based skeletal muscle quantification, high proportion of low-density muscle was an independent poor prognostic indicator of 3-year DFS rate.
Our research team developed an automatic segmentation method using machine learning algorithm for skeletal muscle, and the result was subsequently classified as low-density muscle (−29 to <30 HU) and high-density muscle (30 to 150 HU) based on radiodensity level. Body tissues can be identified based on their radiological attenuation characteristics measured on CT images. The known HU values for body composition are −29 HU to 150 HU for skeletal muscle and −190 to −30 HU for adipose tissue. The presence of excess fat in skeletal muscle can be detected as low attenuation owing to the unique low attenuation value of adipose tissue compared to lean soft tissue. The radiological density of muscle decreases with an increase in the fat content within muscle tissue. Therefore, low muscle radiodensity is a feature of myosteatosis that can be measured non-invasively using CT images. Based on these observations, we aimed to evaluate the predictive value of CT-determined radiodensity-based detailed skeletal muscle quantification on prognosis after curative resection of NSCLC, we found that the presence of healthy or unhealthy muscle was one of the independent factors to predict 3-year DFS rate. The findings of our study are consistent with findings in a previous study, which revealed that low skeletal muscle density was associated with poorer survival in advanced NSCLC, although skeletal muscle mass quantification itself was not an independent prognostic factor in the patient cohort (13). It is assumed that qualitative change was preceded by quantitative change, and the radiodensity based detailed skeletal muscle quantification might be an early biomarker for cancer cachexia syndrome. Although the presence of healthy muscle showed increased DFS, but no significant impact was observed on OS. This may have been due to the limitations of our small sample size and the number of deaths (n=39, 14.3%) that occurred during the study period.
Conclusions
In conclusion, healthy or unhealthy muscle defined by the proportion of low-density skeletal muscle is associated with DFS in resected lung cancer patients. Radiodensity-based detailed skeletal muscle quantification may be an early biomarker to detect the risk of cancer cachexia syndrome and to predict prognosis in patients who plan to undergo curative surgery for NSCLC. Further studies are warranted in oncologic patients to elucidate the mechanisms regarding the radiodensity based detailed quantification of skeletal muscle at all stages of cancer cachexia.
Acknowledgments
Funding: This research was supported by MD-PhD research through the Korea Research-Driven Hospital (grant 2018-5287) and the National Research Foundation of Korea (grant NRF-2018R1C1B5086352).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-2344
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-2344
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-2344). The authors have no conflicts of interest to declare.
Ethics Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The current study was approved by the institutional review board of Gachon University Gil Medical Center (approval number: GCIRB 2020-251). The requirement for informed consent is waived by the ethics review board due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489-95. [Crossref] [PubMed]
- Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539-47. [Crossref] [PubMed]
- Collins J, Noble S, Chester J, et al. The assessment and impact of sarcopenia in lung cancer: a systematic literature review. BMJ Open 2014;4:e003697. [Crossref] [PubMed]
- Kim EY, Kim YS, Park I, et al. Prognostic Significance of CT-Determined Sarcopenia in Patients with Small-Cell Lung Cancer. J Thorac Oncol 2015;10:1795-9. [Crossref] [PubMed]
- Kim EY, Lee HY, Kim KW, et al. Preoperative Computed Tomography-Determined Sarcopenia and Postoperative Outcome After Surgery for Non-Small Cell Lung Cancer. Scand J Surg 2018;107:244-51. [Crossref] [PubMed]
- Deng HY, Hou L, Zha P, et al. Sarcopenia is an independent unfavorable prognostic factor of non-small cell lung cancer after surgical resection: A comprehensive systematic review and meta-analysis. Eur J Surg Oncol 2019;45:728-35. [Crossref] [PubMed]
- Edge S, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th ed. New York: Springer, 2009.
- Anwar SM, Majid M, Qayyum A, et al. Medical Image Analysis using Convolutional Neural Networks: A Review. J Med Syst 2018;42:226. [Crossref] [PubMed]
- Prado CM, Baracos VE, McCargar LJ, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 2009;15:2920-6. [Crossref] [PubMed]
- Meza-Junco J, Montano-Loza AJ, Baracos VE, et al. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol 2013;47:861-70. [Crossref] [PubMed]
- Fukushima H, Yokoyama M, Nakanishi Y, et al. Sarcopenia as a prognostic biomarker of advanced urothelial carcinoma. PLoS One 2015;10:e0115895. [Crossref] [PubMed]
- Yang M, Shen Y, Tan L, et al. Prognostic Value of Sarcopenia in Lung Cancer: A Systematic Review and Meta-analysis. Chest 2019;156:101-11. [Crossref] [PubMed]
- Sjoblom B, Gronberg BH, Wentzel-Larsen T, et al. Skeletal muscle radiodensity is prognostic for survival in patients with advanced non-small cell lung cancer. Clin Nutr 2016;35:1386-93. [Crossref] [PubMed]


