
High serum C-reactive protein levels predict survival in patients with treated advanced lung adenocarcinoma
Introduction
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKIs) have been developed as new therapeutic agents for lung cancers. Since gefitinib became available for general clinical use in 2002 (1,2), much information has been collected on molecularly-targeted therapeutic agents. It has been reported that EGFR mutations are predictors of susceptibility to gefitinib (3). Compared with cytotoxic chemotherapy, first-line treatment with gefitinib extended progression-free survival (PFS) with tolerable toxicity in patients with EGFR mutations (4,5). EGFR mutated non-small cell lung cancer (NSCLC) has also been treated with other EGFR-TKIs including erlotinib (6,7), afatinib (8,9), osimertinib (10), and dacomitinib (11,12) as a first-line chemotherapy. The presence or absence of EGFR gene mutations is an important prognostic factor in advanced NSCLC.
The evidence implies a strong relationship between cancer and inflammation (13). C-reactive protein (CRP) level is a marker for systemic inflammation, and high serum CRP levels (CRP ≥10 mg/L) were reported to predict resistance to gefitinib (14) and erlotinib therapy (15). However, in these studies, EGFR-TKI treatment was performed, regardless of the EGFR mutation status and included many patients who had previously undergone cytotoxic chemotherapy. These factors do not match the current clinical practice. To reflect modern practices, we investigated the clinical utility of serum CRP levels measured before the start of EGFR-TKI as a first-line chemotherapy in EGFR mutated NSCLC. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-3123).
Methods
Patients and clinical characteristics
We retrospectively investigated patients referred for lung cancer treatment at Shimane University Hospital between March 2010 and December 2018. All included patients had lung cancer at an advanced stage for which radical treatment was not possible. The following variables were collected for the purpose of analysis: age, sex, smoking status, tumor histology (adenocarcinoma), Charlson Comorbidity Index (CCI), stage (according to the seventh edition of the TNM Classification), Eastern Cooperative Oncology Group performance status (ECOG PS), chemotherapy regiment, EGFR mutation status and serum CRP levels. Blood sampling was performed as part of routine diagnostic procedures. Serum CRP levels were recorded from the date closest to the date of diagnosis. Most data points were from the day of biopsy. If there were no data within two weeks from the date of diagnosis, it was set as missing data. Patients who had missing data, gene mutation except EGFR were excluded.
We analyzed patient and tumor characteristics to identify factors associated with PFS and overall survival (OS). If the exact date of death was unavailable, OS was calculated from the date of diagnosis until either death due to any cause or final follow-up. PFS was defined as the period from diagnosis to the radiological progression of disease or death. Data on radiological responses and dates of progression were obtained from the medical records as they were documented at the time by the treating physician according to his/her assessment. Date of death was also obtained from the medical records. Patients who were selected for best supportive care were excluded from OS and PFS analysis. Patients who had EGFR mutated adenocarcinoma not treated with EGFR-TKI monotherapy were excluded from OS and PFS analysis.
Our research complies with the ethics guidelines by the local ethics committee of Shimane University. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study approved by the Institutional Review Board (2019-1218-1) and the informed consents were waived due to the retrospective nature of the study.
Detection of EGFR mutation
Tumor specimens were collected by bronchoscopy, computed tomography guided biopsy, pleural effusion cytology or surgical procedures. EGFR mutational analysis was performed using peptide nucleic acid-locked nucleic acid polymerase chain reaction (PCR) clamp or real time PCR (cobas® EGFR Mutation Test v2).
Statistical analyses
Statistical analyses were performed in the GraphPad Prism 7 software program (GraphPad Software, La Jolla, CA, USA) and the R (version 3.6.2, R Foundation for Statistical Computing, Vienna, Austria). Qualitative variables are reported as frequency and percentage and quantitative variables as mean and range. For comparisons between two groups, non-normally distributed data were assessed using the Mann-Whitney test. Categorical data were analyzed using Fisher’s exact test. Receiver operating characteristic (ROC) curves or Youden’s index was used to determine the best cutoff values for CRP levels as a prognostic factor. PFS and OS were estimated using Kaplan-Meier analysis. Hazard ratio (HR)s and their confidence interval (CI)s were calculated using univariable and multivariable Cox proportional hazard model. All statistical tests used in this study were two-sided. Statistical significance was defined as a P value <0.05.
Results
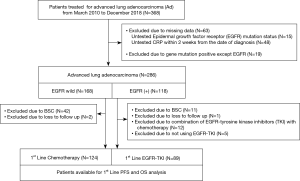
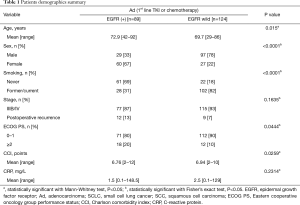
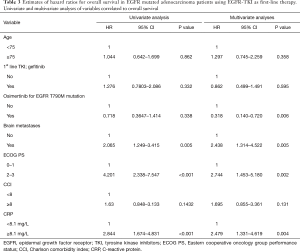
The study flowchart is shown in Figure 1. Of the 286 total cases of advanced lung adenocarcinoma, 213 [EGFR+ (n=168), EGFR wild (n=118)] were included to analyze PFS and OS. Nineteen cases with known positive mutations other than EGFR were excluded. Demographic data of all included patients are shown in Table 1. Patients with wild-type EGFR tended to have poor ECOG PS and high CCI, but there was no difference in mean serum CRP levels relative to the patients with mutant EGFR.
Full table
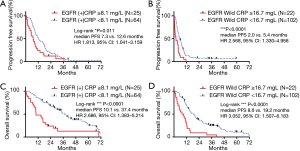
The best cutoff points of CRP levels as determined by ROC curve or Youden’s index were 8.1 mg/L (EGFR+) and 16.7 mg/L (EGFR wild), respectively. Kaplan-Meier analyses compared patients with high CRP levels with those with normal CRP levels (Figure 2). Patients with high CRP levels had significantly shorter PFS than those with normal CRP levels [Figure 2A: EGFR (+), median 7.3 versus 12.6 months, HR 1.813, 95% CI: 1.041–3.159, P=0.011; Figure 2B: EGFR (−), median 2.0 versus 5.4 months, HR 2.568, 95% CI: 1.330–4.958, P<0.0001]. Similar to PFS, OS was shorter in the adenocarcinoma subtype in patients with high CRP levels [Figure 2C: EGFR (+), median 10.1 versus 37.4 months, HR 2.686, 95% CI: 1.383–5.214, P<0.0001; Figure 2D: EGFR (−), median 8.6 versus 19.2 months, HR 3.052, 95% CI: 1.507–6.183, P<0.0001).
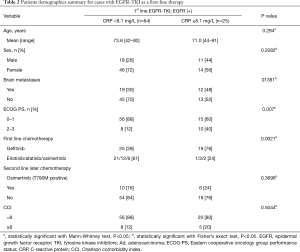
Characteristics of patients in the EGFR mutated adenocarcinoma group are shown in Table 2 for each serum CRP level. At high CRP levels, gefitinib was the most frequent first-line chemotherapy. The ECOG PS 2–3 case ratio was high. We performed Cox regression analysis of the available data of 89 patients to determine the correlation between therapeutic efficacy of EGFR-TKIs and clinical factors such as age (<75 vs. ≥75 years), first-line EGFR-TKI (gefitinib vs. others), use of osimertinib for T790M mutations, brain metastases status (no vs. yes), ECOG PS (0–1 vs. 2–3), CCI (<8 vs. ≥8) and serum CRP level (<8.1 vs. ≥8.1 mg/L) (Table 3). Among these factors, having brain metastases (HR 2.065; 95% CI: 1.249–3.415; P=0.005), ECOG PS 2–3 (HR 4.201; 95% CI: 2.338–7.547; P<0.001) and high serum CRP level (HR: 2.844; 95% CI: 1.674–4.831; P<0.001) had significant negative prognostic factors for survival in univariate analysis. Brain metastases (HR: 2.438; 95% CI: 1.314–4.522; P=0.005), ECOG PS 2–3 (HR: 2.744; 95% CI: 1.453–5.180; P=0.002), and high CRP levels (HR: 2.479; 95% CI: 1.331–4.619; P=0.004) were significant and independent negative prognostic factors for OS according to the multivariate analysis. The use of osimertinib for the EGFR T790M mutation (HR: 0.318; 95% CI: 0.140–0.720; P=0.006) was a significant positive prognostic factor for OS in the multivariate analysis.
Full table
Full table
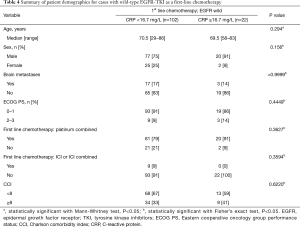
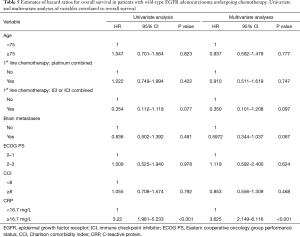
Characteristics of patients in the EGFR wild-type adenocarcinoma group are shown in Table 4 for each serum CRP level. The EGFR wild-type adenocarcinoma group were investigated for history of platinum and immune checkpoint inhibitor (ICI) use. Only high CRP levels contributed to prognosis with significant differences in both univariate and multivariate analysis (Table 5).
Full table
Full table
Discussion
Our present study suggested that serum CRP is clinically relevant in patients with advanced lung adenocarcinoma. Especially for high serum CRP levels can be expected shorter PFS and OS. This tendency was present even if EGFR mutation was positive.
Similar findings have been reported by others (14,15). The strength of the present research is that it only examined EGFR mutation-positive cases and cases in which EGFR-TKI was used as a first-line treatment in compliance with current clinical practices. CRP level is a prognostic factor for survival in patients with inoperable NSCLC (16-18). These studies were performed in the context of non-small cell carcinoma and include SCC. The results of the present study indicated that CRP level was a useful indicator in adenocarcinoma. Since a different treatment method is selected for squamous cell lung carcinoma than for adenocarcinoma, showing data only for adenocarcinoma is a strength of this study.
The modified Glasgow Prognostic Score (mGPS), which uses CRP, represents not only host systemic inflammatory response status but also nutritional status (19). mGPS is categorized into three classes based on CRP and serum albumin concentrations. Patients with high CRP level (≥10 mg/L) and hypoalbuminemia (<3.5 g/dL), those with only high CRP levels (≥10 mg/L), and those with normal CRP levels (<10 mg/L) with or without hypoalbuminemia were categorized as 2, 1, and 0 mGPS, respectively. mGPS =2 is a prognosis predictor of lung adenocarcinoma without driver mutation (20). In the present study, the CRP cutoff was also set to 16.7 mg/L, and the prognosis in adenocarcinoma without EGFR mutation could be predicted.
Our study was limited by the small sample size. Grouping patients by histologic subtype and EGFR mutation status reduces the sample size, but at the same time, it has the advantage of reflecting the actual clinical situation. Further studies with a bigger sample size are needed to ensure statistical reliability. Although the biomarkers were derived, the present study is limited by being a single-center retrospective study.
Conclusions
CRP level is used as a regular prognosis test, but it is a good prognostic factor only under the following conditions: (I) the cancer subtype is adenocarcinoma and (II) the treatment approach used is chemotherapy. Even if EGFR-TKI, which has a very strong therapeutic effect, is used, CRP alone can predict the therapeutic effect and prognosis. In EGFR wild-type adenocarcinoma, CRP level may reflect the therapeutic effect and prognosis better than the ECOG PS or chemotherapy regimen.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-3123
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-3123
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-20-3123
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-3123). YT reports personal fees from Astrazeneca, personal fees from Daiichi Sankyo Co., Ltd., personal fees from Chugai Pharmaceutical Co., Ltd., outside the submitted work. TI reports personal fees from Boehringer-ingelheim, personal fees from Astrazeneca, personal fees from Pfizer, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study complies with ethics guidelines by the local ethics committee of Shimane University. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study approved by the Institutional Review Board (2019-1218-1) and the informed consents were waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer. J Clin Oncol 2003;21:2237-46. [Crossref] [PubMed]
- Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: A randomized trial. JAMA 2003;290:2149-58. [Crossref] [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018;378:113-125. [Crossref] [PubMed]
- Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): A randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:1454-66. [Crossref] [PubMed]
- Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non-small-cell lung cancer and EGFR-activating mutations. J Clin Oncol 2018;36:2244-50. [Crossref] [PubMed]
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860-7. [Crossref] [PubMed]
- Masago K, Fujita S, Togashi Y, et al. Clinical significance of pretreatment C-reactive protein in patients with advanced nonsquamous, non-small cell lung cancer who received gefitinib. Oncology 2010;79:355-62. [Crossref] [PubMed]
- Fiala O, Pesek M, Finek J, et al. High serum level of C-reactive protein is associated with worse outcome of patients with advanced-stage NSCLC treated with erlotinib. Tumour Biol 2015;36:9215-22. [Crossref] [PubMed]
- Wilop S, Crysandta M, Bendela M, et al. Correlation of C-Reactive protein with survival and radiographic response to first-line platinum-based chemotherapy in advanced non-small cell lung cancer. Onkologie 2008;31:665-70. [PubMed]
- Koch A, Fohlin H, Sörenson S. Prognostic significance of C-reactive protein and smoking in patients with advanced non-small cell lung cancer treated with first-line palliative chemotherapy. J Thorac Oncol 2009;4:326-32. [Crossref] [PubMed]
- Gagnon B, Abrahamowicz M, Xiao Y, et al. Flexible modeling improves assessment of prognostic value of C-reactive protein in advanced non-small cell lung cancer. Br J Cancer 2010;102:1113-22. [Crossref] [PubMed]
- Leung EY, Scott HR, McMillan DC. Clinical utility of the pretreatment Glasgow prognostic score in patients with advanced inoperable non-small cell lung cancer. J Thorac Oncol 2012;7:655-62. [Crossref] [PubMed]
- Minami S, Ihara S, Kim SH, et al. Lymphocyte to monocyte ratio and modified Glasgow prognostic score predict prognosis of lung adenocarcinoma without driver mutation. World J Oncol 2018;9:13-20. [Crossref] [PubMed]


