
Is the size of the lepidic component negligible when measuring the size of the tumor to determine the stage of lung adenocarcinoma?
Introduction
The lung cancer staging system was changed in 2017, and the biggest change was how the tumor size is measured. In the 8th edition TNM classification, the tumor (T) stage is determined by the size of invasive component of primary tumor (1,2). Previously, the T stage was determined by the maximum size of the tumor including the lepidic component (total tumor size). In the latest (8th) edition, the T stage is determined according to the maximum size of the invasive component, without the lepidic component (invasive component size) (3). This difference in classification is an important change for staging early lung adenocarcinoma, which often contains large amounts of lepidic components.
Lung adenocarcinoma is the most common histologic type of primary lung cancer (4). According to the 2015 World Health Organization (WHO) classification of lung tumors, most lung adenocarcinomas are composed of five major components (acinar, papillary, micropapillary, solid, and lepidic) combined in various proportions (5). Of these, the lepidic component is regarded as a component without invasiveness. Adenocarcinoma that contains high levels of the lepidic component has a low malignant potential, and is known to have a good prognosis (6,7). Stage I lung adenocarcinoma often contains a lepidic component. Therefore, in the case of adenocarcinoma containing a large amount of lepidic component, the total tumor size and the invasive component size differ significantly. Even if the total tumor size is very large, if the invasive component size is small, it is classified as T1a. Therefore, it is possible that the lepidic component size can be completely ignored when staging is determined. However, there has been little research done on whether the lepidic component size can be ignored.
The aim of this study was to compare the effects of invasive component size and total tumor size on the prognosis of early lung adenocarcinoma. Thus, when determining the stage of lung cancer, we considered whether the size of the lepidic component can be ignored, while using only the invasive component size as the T descriptor. In addition, we tried to evaluate the prognosis of a large-sized (>3 cm) lung adenocarcinoma previously classified as T2a (stage IB) when it was reclassified as stage IA because of the small invasive component size.
Most of the retrospective studies on lung cancer have used the 8th edition of the TNM staging system without distinguishing the tumor size from the total tumor size and the invasive component size. In order to apply the patients’ data before 2017 to the 8th edition of the TNM staging system, the tumor size of the pathologic specimens had to be re-measured, taking into account the invasive component size. In this study, pathologic specimens of stage I lung adenocarcinoma were re-evaluated from 2010, and the invasive component size was re-measured. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-2963).
Methods
Patients
From 2010 to 2018, 1994 patients underwent curative surgery at a tertiary hospital in Korea. Among those patients, 914 patients who had undergone complete resections were diagnosed as having stage 0 or stage IA lung adenocarcinoma. A total of 301 patients who had undergone sublobar resection were excluded, to eliminate the bias of surgical procedure. To reduce the selection bias, all data were obtained from consecutive patient data. Patients who had received neoadjuvant or adjuvant chemotherapy were not included. Finally, 613 consecutive patients were evaluated retrospectively. The recurrence-free survival (RFS) rate and disease specific survival (DSS) rate were analyzed according to the stage (stage 0, stage IA1 to IA3). Statistical analysis was conducted to find out whether the recurrence of stage IA lung adenocarcinoma was more affected by total tumor size (including lepidic component size) or invasive component size. We also compared the prognosis of large adenocarcinoma (total tumor size >3 cm) with others (total tumor size ≤3 cm) in tumors with the same invasive component size.
Histopathology and re-staging
All surgical specimens were prepared and re-evaluated by board certified pathologists. Tumors were restaged according to the 8th edition of TNM classification (8) by measuring the greatest dimension of the invasive component on the pathology specimen (1). Total tumor size was defined as the greatest dimension of the tumor including the lepidic component. The invasive component size, instead, was defined as the greatest dimension of the invasive component excluding the lepidic component of the tumor. Pathology reports were reviewed for tumor location, lymph node status, visceral pleural invasion, and lymphovascular invasion.
Statistical analysis
The Kaplan-Meier method was used to analyze data collected from the interval between the time of operation and the time of the last follow-up visit. RFS rates and DSS rates according to the TNM stages were estimated by the Kaplan-Meier method. A Cox proportional hazards model was used in a multivariate analysis to identify risk factors for recurrence after surgery in stage IA lung adenocarcinoma. All variables with P<0.10 on univariate analysis were entered into the multivariate analysis. P values of less than 0.05 were considered statistically significant.
Clinicopathological factors were compared between large adenocarcinomas (total tumor size >3 cm) and others (total tumor size ≤3 cm) in the same stage using the Student’s t test or the Wilcoxon rank sum test for continuous variables, and chi-squared or Fisher exact test for categorical variables. The RFS and DSS in both groups were compared using the log-rank test. Statistical analyses were performed using SPSS version 24.0 software (IBM Corp, Armonk, NY, United States).
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the institutional review board of Seoul St. Mary’s Hospital at the Catholic University of Korea and individual consent was waived (Referral number: KC20RISI0430).
Results
Total tumor size versus invasive component size as a risk factor for recurrence
Table 1 shows the clinicopathological characteristics of patients with stage 0 and stage IA lung adenocarcinoma who have undergone anatomical lobectomy. The number of patients with stage 0, stage IA1, stage IA2, and stage IA3 were 13 (2.1%), 222 (36.2%), 262 (42.7%), and 116 (18.9%), respectively. The mean total tumor size (including lepidic component) was 2.0 cm, and the mean invasive component size was 1.3 cm.
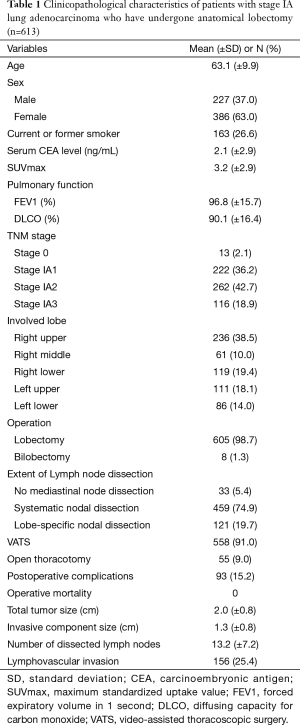
Full table
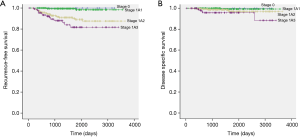
The median follow-up time of the study patients was 1,456 days (34–3,652 days). Recurrence was identified in 40 patients (Table 2). The 5-year RFS rates of stage 0, stage IA1, stage IA2, and stage IA3 were 100%, 98.4%, 89.1%, and 81.7%, respectively (Figure 1A). The 5-year DSS rate of stage 0, stage IA1, stage IA2, and stage IA3 were 100%, 99.3%, 96.9%, and 95.7%, respectively (Figure 1B).
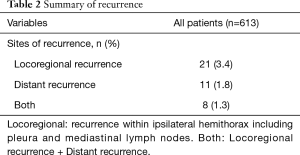
Full table
Univariate and multivariate analyses were conducted to identify the risk factors for recurrence (Table 3). The specific variables identified as significant (P<0.1) by univariate analysis included sex, smoking status, serum carcinoembryonic antigen (CEA) level, maximum standardized uptake value (SUVmax) on positron emission tomography, total tumor size, invasive component size, and lymphovascular invasion (Table 3). These variables were entered into the multivariate model, and two multivariate analyses were conducted according to the method of measuring tumor size: multivariate analysis T, which adopted total tumor size (Table 3); and multivariate analysis I, which adopted invasive component size (Table 3). In multivariate analysis T, total tumor size was not a significant risk factor for recurrence. Conversely, invasive component size was a significant risk factor for recurrence (Hazard ratio =1.658, P=0.043) in multivariate analysis I.
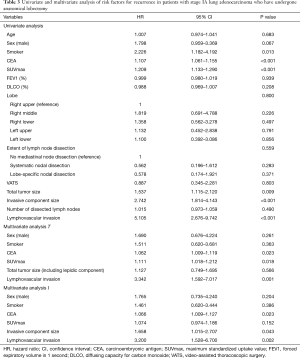
Full table
Comparing tumors with a total tumor size >3 cm and a total tumor size ≤3 cm in stage IA2
Comparisons were not performed in stage 0 and stage IA1 due to the small sample size of tumors with total tumor size ≥3 cm (0 patients of stage 0, 6 patients of stage IA1). Thus, the analysis was performed on tumor samples of stage IA2 and stage IA3.
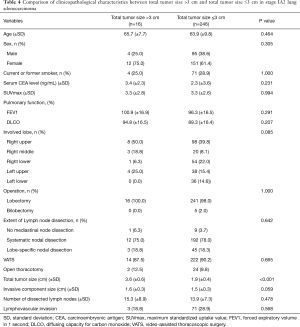
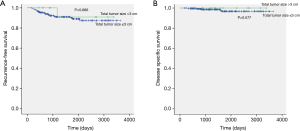
The clinicopathological characteristics were not significantly different between tumors with a total tumor size >3 cm and a total tumor size ≤3 cm in stage IA2 adenocarcinoma (Table 4). The mean total tumor sizes were different in both groups (3.6 and 1.9 cm, P<0.001), but the invasive component size was not statistically different (1.6 and 1.5 cm, P=0.059). The median follow-up time of stage IA2 patients was 1,428 days (173–3,652 days). The 5-year RFS rates of tumors with total tumor size >3 cm and total tumor size ≤3 cm in stage IA2 lung adenocarcinoma were 90.9% and 89.0%, respectively (P=0.686; Figure 2A). The 5-year DSS rates of these groups were 100% and 96.7%, respectively (P=0.577; Figure 2B). There were no statistical differences in the RFS and DSS between these groups.
Full table
Comparing tumors with a total tumor size >3 cm and a total tumor size ≤3 cm in stage IA3
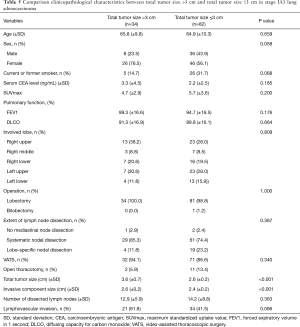
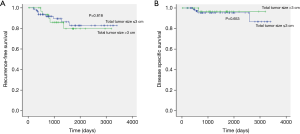
The clinicopathological characteristics were not significantly different between tumors with a total tumor size >3 cm and a total tumor size <3 cm in stage IA3 adenocarcinoma (Table 5). The mean total tumor sizes were 3.6 and 2.6 cm, respectively (P<0.001). The mean invasive component sizes were 2.6 and 2.4 cm, respectively (P<0.001). The median follow-up time of stage IA3 patients was 1,113 days (34–3,407 days). The 5-year RFS rates of tumors with total tumor size >3 cm and total tumor size ≤3 cm in stage IA3 lung adenocarcinoma were 79.7% and 82.8%, respectively (P=0.818; Figure 3A). The 5-year DSS rates of these groups were 96.6% and 95.4%, respectively (P=0.653; Figure 3B). There were no statistical differences in the RFS and DSS between these groups.
Full table
Discussion
The most important characteristic of lung adenocarcinoma is histologic heterogeneity (9). Among the histologic components of adenocarcinoma, the lepidic component is regarded as a non-invasive component of tumors. Therefore, referring to the 8th edition of the TNM staging system, the size of the lepidic component was ignored and only the invasive component size was used as the T descriptor (2,10). However, if the total tumor size including the lepidic component is large, it is debatable whether the total tumor size can be completely ignored. In particular, the prognosis of tumors with a total tumor size greater than 3 cm in those classified as T1a due to the small invasive component size was an important concern of this study. Previously, these tumors were all classified as T2a and diagnosed as stage IB (11). Few studies have addressed this change in classification and the impact on diagnosis and prognosis.
Survival rates were clearly distinguished from Stage 0 to Stage 1A3 in this study when staging was performed using the invasive component size as the T descriptor. Moreover, in multivariate analysis to find risk factors for recurrence, total tumor size was not a risk factor for recurrence, whereas invasive component size was a statistically significant risk factor for recurrence. This confirms that staging by referring only to the invasive component size better reflects prognosis. Moreover, the prognosis did not differ even when the total tumor size was greater than 3 cm in stages IA2 and IA3. This also supports staging by only using the invasive component size, while the size of the lepidic component does not need to be considered at all.
In this study, when performing multivariate analysis to find risk factors for recurrence, two multivariate analyses were performed according to the method of measuring tumor size. In the multivariate analysis T using total tumor size as a variable, CEA, SUVmax, and lymphovascular invasion were risk factors for recurrence. Furthermore, CEA, invasive component size, and lymphovascular invasion were risk factors for recurrence in the multivariate analysis I using invasive component size as a variable. As multivariate analysis was performed using invasive component size as a variable instead of total tumor size, CEA and lymphovascular invasion were still risk factors for recurrence, but SUVmax was not a risk factor for recurrence. This is because tumors with a large total tumor size, containing large amount of lepidic components, usually have low values of SUVmax. Therefore, the relevance of SUVmax is reduced when using the invasive component size as a variable. Although studies have mentioned SUVmax as a predictor of lung cancer prognosis or lymph node metastasis (12-15), the role of SUVmax is estimated to decrease after staging using invasive component size (16). It is well-established from previous studies that CEA and lymphovascular invasion are risk factors for early lung cancer (17-21), and these were also identified as risk factors in this study.
This study only included stage 0 to stage IA3 tumors. The reason for not including stage IB is that it was not sufficient to evaluate the effect of tumor size, as stage IB tumors are affected by visceral pleural invasion as well as by invasive component size. In order to evaluate the effect of the invasive component size and lepidic component size, it was considered appropriate to target only stage 0 to stage IA3.
When selecting a surgical method for stage I lung cancer, sublobar resection can only be considered when the total tumor size is 2 cm or less. In most studies of sublobar resection, total tumor size is used as the determining factor for surgery (22-24). In addition, two randomized trials (CALGB 140503 and JCOG 0802) are ongoing to investigate the hypothesis that sublobar resection is comparable to lobectomy for small-sized (≤2 cm) non-small-cell lung carcinoma (25,26), where tumor size was based on total tumor size. However, in these studies, it was found that the invasive component size better reflects the prognosis than the total tumor size. Another study also showed that T-stage with an invasive component size could better predict the prognosis than T-stage with total tumor size (10). Therefore, for predicting outcome, it is better to conduct a study on sublobar resection with invasive component size, not total tumor size (27). Our previous study reported that prognoses following wedge resection and lobectomy of tumors with an invasive component size smaller than 1 cm were comparable, regardless of the total tumor size (28). Therefore, studies on lung cancer prognosis or sublobar resection should be performed using invasive component size only.
This investigation has some limitations. Firstly, it was a retrospective study. Secondly, we obtained data from a single institution and the sample size was relatively small, so it is difficult to generalize our results. However, this study is meaningful in that the invasive component size was re-measured using the stored pathological specimens. This has not been carried out by previous studies and in doing so we have tried to gain more accurate results. Furthermore, this study examined data from surgical patients treated by a relatively standardized protocol at our institution, a tertiary hospital in Korea. A detailed analysis was also possible because of the information stored in the electronic medical records; we believe that our data can be used as the basis for future investigations. However, a larger study should be performed to validate our results. Finally, this study was based on pathologic findings and did not analyze the relationship with preoperative radiologic discoveries. Therefore, it is not known whether the results of this study can be applied to the size observed by radiologic findings. It would thus be valid to compare the total nodule size and the solid component size by conducting research using radiologic findings in the future.
In conclusion, invasive component size was a risk factor for recurrence of stage IA lung adenocarcinoma, while total tumor size was not a risk factor. Even when the total tumor size was large, there was no difference in prognosis if the size of the invasive component was the same. Therefore, it seems to be appropriate to disregard the size of the lepidic component, and T staging can be assessed by means of the size of the invasive component only. It is expected that more accurate results can be obtained if more data is gathered in the future.
Acknowledgments
A native English-speaking professional (BioMed Proofreading, LLC) refined the written content.
Funding: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2019R1G1A1099670).
Footnote
Reporting Checklist: The author has completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-2963
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-2963
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-2963). Dr. YM reports grants from National Research Foundation of Korea (NRF), during the conduct of the study.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the institutional review board of Seoul St. Mary’s Hospital at the Catholic University of Korea and individual consent was waived (Referral number: KC20RISI0430).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1204-23.
- Rami-Porta R, Asamura H, Travis WD, Rusch VW. Lung cancer - major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:138-55.
- Detterbeck FC. The eighth edition TNM stage classification for lung cancer: What does it mean on main street? J Thorac Cardiovasc Surg 2018;155:356-9.
- Parkin DM, Ferlay J, Curado MP, et al. Fifty years of cancer incidence: CI5 I-IX. Int J Cancer 2010;127:2918-27. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Sasada S, Nakayama H, Miyata Y, et al. Comparison of malignant grade between pure and partially invasive types of early lung adenocarcinoma. Ann Thorac Surg 2015;99:956-60. [Crossref] [PubMed]
- Moon Y, Sung SW, Lee KY, et al. The importance of the lepidic component as a prognostic factor in stage I pulmonary adenocarcinoma. World J Surg Oncol 2016;14:37. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Kameda K, Eguchi T, Lu S, et al. Implications of the Eighth Edition of the TNM Proposal: Invasive Versus Total Tumor Size for the T Descriptor in Pathologic Stage I-IIA Lung Adenocarcinoma. J Thorac Oncol 2018;13:1919-29.
- Moon Y, Choi SY, Park JK, et al. Prognostic factors in stage IB non-small cell lung cancer according to the 8(th) edition of the TNM staging system after curative resection. J Thorac Dis 2019;11:5352-61. [Crossref] [PubMed]
- Nair VS, Krupitskaya Y, Gould MK. Positron emission tomography 18F-fluorodeoxyglucose uptake and prognosis in patients with surgically treated, stage I non-small cell lung cancer: a systematic review. J Thorac Oncol 2009;4:1473-9. [Crossref] [PubMed]
- Moon Y, Kim KS, Lee KY, et al. Clinicopathologic Factors Associated With Occult Lymph Node Metastasis in Patients With Clinically Diagnosed N0 Lung Adenocarcinoma. Ann Thorac Surg 2016;101:1928-35. [Crossref] [PubMed]
- Ghaly G, Rahouma M, Kamel MK, et al. Clinical Predictors of Nodal Metastases in Peripherally Clinical T1a N0 Non-Small Cell Lung Cancer. Ann Thorac Surg 2017;104:1153-8. [Crossref] [PubMed]
- Ye B, Cheng M, Li W, et al. Predictive factors for lymph node metastasis in clinical stage IA lung adenocarcinoma. Ann Thorac Surg 2014;98:217-23. [Crossref] [PubMed]
- Moon Y, Choi SY, Park JK, et al. Risk Factors for Occult Lymph Node Metastasis in Peripheral Non-Small Cell Lung Cancer with Invasive Component Size 3 cm or Less. World J Surg 2020;44:1658-65. [Crossref] [PubMed]
- Kuo SW, Chen JS, Huang PM, et al. Prognostic significance of histologic differentiation, carcinoembryonic antigen value, and lymphovascular invasion in stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2014;148:1200-7.e3. [Crossref] [PubMed]
- Mollberg NM, Bennette C, Howell E, et al. Lymphovascular invasion as a prognostic indicator in stage I non-small cell lung cancer: a systematic review and meta-analysis. Ann Thorac Surg 2014;97:965-71. [Crossref] [PubMed]
- Park C, Lee IJ, Jang SH, et al. Factors affecting tumor recurrence after curative surgery for NSCLC: impacts of lymphovascular invasion on early tumor recurrence. J Thorac Dis 2014;6:1420-8. [PubMed]
- Moon Y, Park JK, Lee KY, et al. Lymphatic invasion is a more significant prognostic factor than visceral pleural invasion in non-small cell lung cancer with tumours of 3 cm or less. Respirology 2017;22:1179-84. [Crossref] [PubMed]
- Maeda R, Suda T, Hachimaru A, et al. Clinical significance of preoperative carcinoembryonic antigen level in patients with clinical stage IA non-small cell lung cancer. J Thorac Dis 2017;9:176-86. [Crossref] [PubMed]
- Zhao ZR, Situ DR, Lau RWH, et al. Comparison of Segmentectomy and Lobectomy in Stage IA Adenocarcinomas. J Thorac Oncol 2017;12:890-6. [Crossref] [PubMed]
- Moon MH, Moon YK, Moon SW. Segmentectomy versus lobectomy in early non-small cell lung cancer of 2 cm or less in size: A population-based study. Respirology 2018;23:695-703. [Crossref] [PubMed]
- Taioli E, Yip R, Olkin I, et al. Survival after Sublobar Resection for Early-Stage Lung Cancer: Methodological Obstacles in Comparing the Efficacy to Lobectomy. J Thorac Oncol 2016;11:400-6. [Crossref] [PubMed]
- Blasberg JD, Pass HI, Donington JS. Sublobar resection: a movement from the Lung Cancer Study Group. J Thorac Oncol 2010;5:1583-93. [Crossref] [PubMed]
- Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [Crossref] [PubMed]
- Moon Y, Park JK, Lee KY. The Effect of Resection Margin Distance and Invasive Component Size on Recurrence After Sublobar Resection in Patients With Small (</=2 Cm) Lung Adenocarcinoma. World J Surg 2020;44:990-7. [Crossref] [PubMed]
- Moon Y, Park JK, Lee KY, et al. Prognosis after wedge resection in patients with 8(th) edition TNM stage IA1 and IA2 non-small cell lung cancer. J Thorac Dis 2019;11:2361-72. [Crossref] [PubMed]


