
Safety and efficacy of an implantable device for management of gastroesophageal reflux in lung transplant recipients
Introduction
Gastroesophageal reflux disease (GERD) is common after lung transplantation (LTx), affecting more than half of LTx recipients (1-3). In addition to typical lifestyle impacts of severe GERD, it may also predispose to micro-aspiration events that contribute to development of chronic lung allograft dysfunction (CLAD), a clinical manifestation of chronic rejection that accounts for significant morbidity and mortality after LTx (4). Pre-clinical studies support an association between GERD and CLAD, albeit not definitively, demonstrating increased inflammation and upregulation of pro-fibrotic mechanisms in lung allografts after aspiration events (5,6). In the clinical setting, the presence of digestive components in bronchoalveolar lavage fluid (BALF) is also associated with heightened incidence and accelerated progression of CLAD (7,8).
Laparoscopic anti-reflux surgery (LARS) has historically been the preferred treatment for post-LTx GERD (9). While anti-secretory medications such as proton pump inhibitors (PPI) raise gastric pH, they do not reduce nonacid reflux events or digestive components in BALF (9,10). Surgical GERD management provides a mechanical barrier to reflux and aspiration which may preserve pulmonary function, reduce CLAD severity (11-14), and improve symptom-related quality of life (15). While laparoscopic fundoplication is an effective treatment for post-LTx GERD, these procedures may be associated with side effects including chronic dysphagia, gas bloating, and inability to belch that may leave patients unsatisfied postoperatively (16,17).
Magnetic sphincter augmentation (MSA) is an emerging minimally invasive surgical technique that relieves GERD symptoms, while avoiding potential side effects of traditional LARS procedures and shortening post-operative hospital stays (16). The LINX Reflux Management System is an MSA device composed of interlinked titanium beads with magnetic cores, placed laparoscopically around the gastroesophageal junction. The beads separate to accommodate a swallowed bolus and then reapproximate to augment the lower esophageal sphincter in the closed position (16). In the general population, the LINX device offers sustained decrease in mean acid exposure time, freedom from daily PPI use, and improved symptom-related quality of life (16,18-20). However, in immunosuppressed patients such as LTx recipients, device implantation is concerning due to risk of severe infection that may compromise long-term outcomes (21). We evaluated the safety and efficacy of the LINX Reflux Management System in this chronically immunosuppressed population. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-3276).
Methods
Study population and design
Patients who underwent isolated LTx followed by post-transplant LINX implantation at Duke University Hospital between 2017 and 2019 were followed prospectively in the Reflux Following Lung Transplantation and Associated Treatment Registry. Follow-up was closed on September 3, 2020. Patients were referred by pulmonary transplant providers to thoracic surgery for evaluation of anti-reflux surgery and then reviewed and assessed by the surgical team. Based on review of physiologic test results, clinical history, and patient preference, patients were offered LINX placement and/or traditional fundoplication. Specifically, patients with normal esophageal motility and no dysphagia were given the option of either LINX placement or fundoplication. Those who preferred to undergo LINX placement subsequently underwent an insurance approval process to determine whether their insurance providers would cover the LINX device; patients whose insurance providers covered the device underwent LINX placement. Alternatively, patients with abnormal esophageal motility, those who preferred to undergo traditional fundoplication, and those who did not have insurance coverage for the LINX device underwent fundoplication. A subset of patients who underwent pre-transplant GERD evaluation were determined to have GERD severe enough to warrant early anti-reflux surgery following LTx based on results of ambulatory pH testing and barium swallow, and additional clinical findings including presence of bronchoscopic or histologic evidence of aspiration. These patients were triaged to anti-reflux surgery within 3 months of LTx without further post-transplant GERD evaluation. Patients who underwent prior fundoplication or alternative anti-reflux procedure were excluded from this registry. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Duke University (Pro00058718) and informed consent was taken from all individual participants.
Patient demographics, medical history, and operative characteristics pertaining to LINX implantation were abstracted from patient charts. Medical history of interest included pre-transplant comorbidities (hypertension, diabetes, tobacco use, GERD), transplant type (single, bilateral), and donor and recipient characteristics including lung allocation score at transplantation, panel reactive antibody at transplantation, cytomegalovirus serostatus, and induction and maintenance immunosuppression. During the study period, LINX implantation was performed by a single surgeon specializing in foregut and lung transplant surgery.
Patients were followed by thoracic surgery and transplant providers at routine clinic visits per standard of care. All patients underwent baseline ambulatory 24-hour pH testing, high-resolution impedance esophageal manometry, and pulmonary function testing (PFT) prior to LINX implantation. During the study period, ambulatory pH testing was conducted using both dual probe pH tests and pH tests with impedance. Patients’ pH test results including acid contact times and DeMeester scores were evaluated in the context of the listed reference ranges for the particular test administered to distinguish normal from abnormal results. Gastric emptying studies, upper endoscopy, and barium swallow were performed at the providers’ discretion. After LINX implantation, follow-up data was recorded at the post-operative visit, 3, 6, and 12 months post-implantation. Use of acid-suppressing medication (PPI or H2 blocker), any episodes of redo LTx or GERD-related reoperation, and recent PFTs were recorded at each follow-up time point and transbronchial lung biopsies were performed per clinical protocol. Unplanned clinic visits during the study period were recorded and adverse events were documented and reviewed. Patients were scheduled for repeat ambulatory pH testing and esophageal manometry as needed based on patient willingness, symptoms, and provider assessments. Date of last follow-up, patient status (alive or deceased), and dates and pathologic grades of any biopsy-proven acute rejection episodes were recorded.
Comparison of LINX and traditional fundoplication
A group of LTx recipients who underwent post-transplant LARS was matched 1:1 (nearest neighbor, caliper =0.31) based on age at anti-reflux surgery (±5 years) and sex. One-year outcomes and change in pulmonary function were compared between LTx recipients who underwent LINX implantation and those who underwent traditional fundoplication.
Statistical analysis
First, we characterized LTx recipients who underwent LINX implantation and evaluated the efficacy of the LINX device. Patient demographics, medical history, and operative characteristics were summarized using medians and interquartile ranges (IQR) for continuous variables and frequencies and proportions for categorical variables. Patient demographics, medical history, and anti-reflux surgery operative characteristics were compared between LINX and fundoplication groups using Wilcoxon rank-sum tests for continuous variables and Chi-squared and Fisher’s exact tests for categorical variables. Among LINX patients, pre- and post-LINX data points were compared using statistical methods for paired data including McNemar’s test for categorical variables (i.e., acid-suppressing medication use) and two-sample paired student’s t tests for continuous variables (i.e., distal acid contact time on ambulatory pH testing).
Second, we compared LTx recipients who underwent LINX implantation and those who underwent traditional LARS and evaluated the safety of the LINX device. One-year patient, rejection-free, side effect-free, and reoperation-free survival were estimated in an unadjusted fashion using the Kaplan-Meier method and compared between groups using log-rank tests. Characteristics of acute rejection episodes before and after anti-reflux surgery were compared between LINX and fundoplication groups using Chi-squared and Fisher’s exact tests. Pulmonary function was assessed based on the change in forced expiratory volume in one second (FEV1) using a linear mixed effects model (fixed effects: time, procedure type; random effects: patient) with an interaction between time and procedure type to evaluate whether the change in FEV1 over time differed between LINX and fundoplication groups. A two-sided P value less than 0.05 was considered statistically significant. All analyses were performed using R version 3.6.1 (Vienna, Austria).
Results
Study population
Overall, 17 LTx recipients who underwent post-transplant LINX implantation were included. Median age at LINX implantation was 61 years (IQR, 44–71 years), 29.4% were female, and 100% were White. Most (76.5%) patients had undergone bilateral LTx, 11 (64.7%) had a history of pre-transplant GERD, and 12 (70.6%) were taking acid-suppressing medication prior to LTx. Patient and LTx characteristics were similar between LINX and fundoplication groups (Table 1).
Full table
Operative characteristics of the LINX implantation
LINX implantation was performed median 1.40 years (IQR, 0.52–2.73 years) post-transplant. Six (35.3%) patients were found to have a hiatal hernia, most of which were documented as “small” (4 out of 6 patients). All procedures were performed laparoscopically; robot-assistance was employed in 3 (17.6%) cases. Two (11.8%) patients underwent paraoesophageal hernia repair and 1 (5.9%) underwent lysis of adhesions concurrent with LINX implantation. Median operative duration was 106 minutes (IQR, 102–139 minutes) and patients remained in the hospital for median 1 day (range, 1–4 days) post-operatively. No intraoperative complications were noted (Table 2).
Full table
Compared to LINX implantation, laparoscopic fundoplication cases were more likely to be performed with robot-assistance (88.2% vs. 17.6%, P<0.01). Postoperative hospital lengths of stay were significantly longer after fundoplication than after LINX implantation [median 2 (range 1–25) vs. 1 (range 1–4) days, P=0.02]. Additional operative characteristics were similar between groups (Table 2).
Efficacy of the LINX device
Ambulatory pH testing
Pre-LINX ambulatory pH testing was performed median 0.21 years (range, −3.53 to 7.16 years) post-transplant; pre-transplant pH tests were used for baseline assessment in three cases as these patients were considered to have severe GERD on pre-transplant evaluation that warranted an early post-LTx GERD surgery. Eight (47.1%) patients underwent follow-up pH testing median 0.62 years (range, 0.19–1.04 years) post-LINX implantation. Overall, 4 out of 8 (50.0%) patients had a reduction in total distal acid contact time, 3 out of 8 (37.5%) achieved normal total distal acid contact times, and 3 out of 8 (37.5%) achieved normal DeMeester scores after LINX implantation [pre-LINX: 1/17 (5.9%) and 2/17 (11.8%), respectively] (Figures 1,2, Table 3). At one year post-LINX implantation, 14 (82.4%) patients remained on acid-suppressing medication at the discretion of their pulmonary transplant teams (pre-LINX: 100%, P=0.3).
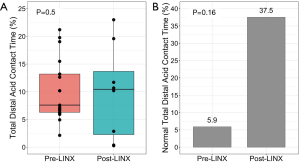
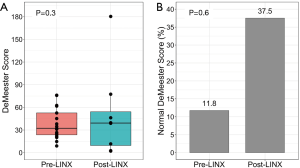

Full table
Esophageal manometry
Pre-LINX esophageal manometry was performed median 0.25 years (range, 0.10–7.17 years) post-transplant. Nine (52.9%) patients underwent follow-up esophageal manometry median 0.65 years (range, 0.19–1.04 years) post-LINX implantation. There were no significant changes in manometric parameters after LINX implantation (Table 4).
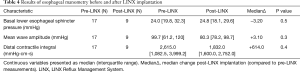
Full table
Safety of the LINX device compared to traditional fundoplication
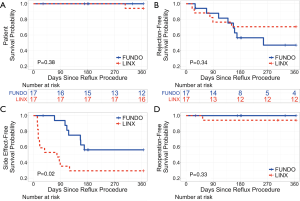
Among patients who underwent LINX implantation, one-year patient, rejection-free, side effect-free, and reoperation-free survival were 94.1%, 70.6%, 29.4%, and 94.1%, respectively. Patient, rejection-free, and reoperation-free survival were similar between LINX and fundoplication groups (all P>0.05). Side effect-free survival was worse among patients who underwent LINX implantation compared to those who underwent traditional fundoplication (P=0.02) (Figure 3). However, in general, side effects occurred early after LINX implantation and resolved over time. The most common side effects were minor including dysphagia, vomiting, residual reflux symptoms, and throat pain. One patient underwent device dilation for persistent dysphagia 55 days post-LINX implantation with subsequent device explant 77 days post-implantation for refractory dysphagia. Patients in both LINX and fundoplication groups experienced postoperative weight fluctuations. By 6 months postoperatively, patients who underwent LINX implantation experienced a median change in body weight of +2.0 vs. −2.7 kg among those who underwent traditional fundoplication (P>0.05). Side effects that occurred among LINX and fundoplication patients during the first year after anti-reflux surgery are summarized in Table 5.
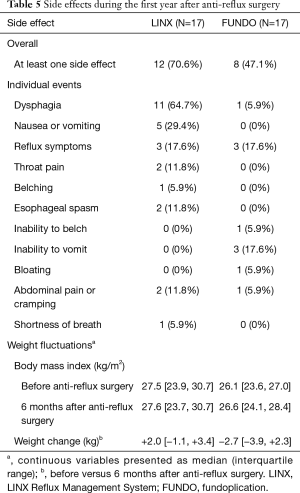
Full table
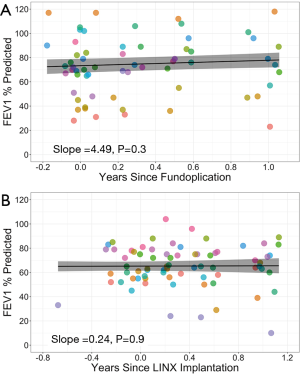
Fourteen (82.4%) patients who underwent LINX implantation and 10 (58.8%) patients who underwent traditional fundoplication had evidence of acute rejection on at least one biopsy prior to anti-reflux surgery (P=0.13). After anti-reflux surgery, 5 (29.4%) LINX and 8 (47.1%) fundoplication patients had at least one rejection episode within one year (P=0.3). The severity of rejection after anti-reflux surgery was graded as minimal (grade A1) or mild (grade A2) in all cases (Table 6). There was no change in FEV1 across pre- and post-operative measurements in either LINX or fundoplication groups (Figure 4). The trajectory of FEV1 over time was similar between groups (interaction P=0.4).
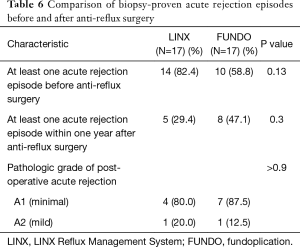
Full table
Discussion
In this study, we explored the safety and early efficacy of the LINX Reflux Management System, a novel implantable anti-reflux device, in LTx recipients. Compared to traditional LARS, the short-term safety of LINX implantation was similar with comparable rates of mortality, acute rejection, and reintervention up to one year post-operatively. As expected from post-market approval studies in non-transplant patients, LINX implantation was associated with significantly more early side effects, primarily dysphagia. In a limited efficacy assessment, a small subset of patients achieved normal esophageal acid exposure time after LINX implantation. Our findings suggest that use of the LINX Reflux Management System in this chronically immunosuppressed population is safe, but further study is required to understand its long-term effectiveness for surgical management of post-transplant GERD.
The efficacy of the LINX device among patients in the general population has previously been reported with outcomes up to 5 years post-implantation detailed in the literature (19,22). As the foundational study in this area, Bonavina et al. conducted a multicenter prospective clinical trial examining use of the LINX device for management of medication-refractory GERD and found that the majority of patients demonstrated a significant decrease in esophageal acid exposure by 3 months after LINX implantation (23). They have since reported outcomes at 1, 2, 4, and 5 years post-LINX implantation, demonstrating a sustained reduction in esophageal acid exposure time throughout the follow-up period with up to 90% of patients achieving normal esophageal acid exposure time by 2 years, maintained by 75% by 5 years (16,18,19). These findings are corroborated by Ganz et al. who report normalization of esophageal acid exposure time in 58% and greater than or equal to 50% reduction of esophageal acid exposure time in 64% of patients by 1 year, the latter increasing to 83% of patients by 5 years (22,24).
Since postoperative patients who are asymptomatic are less likely to submit to repeat ambulatory pH testing, post-LINX acid exposure times were available for only 37.5% of patients in our study. Nevertheless, within that group 50% had a reduction in total distal acid contact times, which is comparable to the studies referenced above. Also, while baseline total distal acid contact times were similar among patients in our study compared to those in the general population (median 7.6% vs. 8.0–11.9%) (16,18,19,23,24), the pathophysiology of GERD in LTx recipients is complex, affected by multiple factors including use of immunosuppressive medications and anatomic considerations such as vagal nerve injury resulting from the transplant operation that are absent among non-transplant patients (1-3,25). In light of these unique circumstances that persist beyond LINX implantation, it may be unreasonable to expect that LTx recipients will achieve normal esophageal acid exposure times, particularly in the short term post-LINX period. Furthermore, while the majority of patients in our study continued acid-suppressing medication after LINX implantation despite resolution of GERD symptoms, this reflects standard practice among the transplant pulmonology providers at our institution, and therefore cannot be used as a reliable indicator of LINX efficacy in this population. This is further complicated by a significant proportion of LTx recipients with GERD who exhibit minimal or no symptoms. Continued long-term follow-up with acquisition of additional post-LINX pH testing is required to better characterize the effects of LINX implantation on esophageal acid exposure in these complex patients. Accordingly, our team has considered increasing use of BRAVO pH studies both pre- and postoperatively, since use of a small capsule rather than a pH probe may reduce patient discomfort and improve willingness to undergo testing at multiple timepoints.
Prior studies have also addressed the safety of the LINX device, particularly surrounding the concern for erosion of the device into the esophagus (23,24). Despite this concern, device erosions and migrations seem to be rare with no occurrences reported during post-implantation follow-up across numerous studies (16,18,19,22-24). At our institution, the LINX device is routinely upsized after measurement of the esophageal circumference. This leaves the device somewhat “loose” at the gastroesophageal junction, but we believe that subsequent scarring secures it in place while at the same time ensuring that device erosion does not occur.
A more legitimate concern post-LINX implantation is the potential for dysphagia. Across previous studies, dysphagia affects between 43–68% of patients early after implantation (16,18,23,24). This problem tends to resolve over time decreasing to approximately 10% at 1 year and less than 5% beyond 2 years (24). Accordingly, persistent dysphagia was the most common indication for reintervention, with patients undergoing device dilation followed by device explant if dilation did not provide adequate symptom relief. Rates of device explant across prior studies ranged from 2–7% (16,18,19,22,23). These findings are reflected in our results, which indicate that dysphagia and vomiting were the most common side effects among patients in our cohort. Nevertheless, for most patients, postoperative dysphagia was transient, resolving spontaneously within one year after LINX implantation. In our study, only one individual (5.9% of patients) underwent device dilation followed by device explant due to persistent dysphagia. That patient had normal manometry prior to implantation of the device; however, a barium swallow suggested mild to moderate esophageal dysmotility with associated tertiary contractions.
Consistent with prior studies, LINX patients at our institution are initiated on a regular diet immediately on postoperative day 1 (16,18,19,23,24). In contrast, fundoplication patients begin with a liquid diet, transitioning to solids later in the recovery period. Indeed, prior work suggests that the LINX device may better support normal eating behavior during the early postoperative period (23), likely facilitating early weight gain among LINX patients in our cohort, compared to weight loss among those undergoing fundoplication. Likewise, in addition to adjusting to the device itself, the high rate of dysphagia associated with LINX implantation may be confounded by differential postoperative management protocols for patients undergoing distinct anti-reflux procedures. Importantly, however, prior work suggests that a central benefit of MSA devices as an alternative to traditional LARS is preserved ability to belch and vomit (16). Indeed, fewer than 5% of patients reported inability to belch or vomit after LINX implantation (18), compared to 34% of dissatisfied post-fundoplication patients (26). Although we did not specifically examine this aspect of post-LINX quality of life and esophageal function in our study, inability to belch or vomit were not specifically noted among LINX patients in our cohort, but were among the most common side effects reported in the fundoplication group, affecting 17.6% of patients during the first post-operative year.
While long-term use of the LINX device has been associated with a satisfactory safety profile within the general population, one primary concern surrounding long-term device implantation in LTx patients is risk of severe infection in the face of chronic immunosuppression. In an institutional study of LTx recipients, Palmer et al. found that post-transplant survival was significantly compromised among patients with documented bacteremia during the post-transplant period, with 44% 3-year survival among patients with an episode of bacteremia versus 71% among those without (P=0.0001) (21). In the present study, there were no documented cases of infection or bacteremia. Once implanted, the LINX device is not exposed to the environment, which may reduce the long-term risk of infection associated with this device. While our findings support the short-term safety of LINX implantation in LTx recipients, a longer duration of follow-up is needed to fully understand the safety of LINX implantation in this population.
There are several limitations in our study that warrant discussion. First, this study examines a small cohort of patients at a single, large academic institution and therefore represents a highly specialized experience that may not be generalizable to other institutions. As additional technologies to treat GERD become more widely utilized, follow-up studies should examine trends in a larger group of LTx recipients that may likewise be better equipped to compare efficacy among novel anti-reflux technologies and traditional LARS procedures. Additionally, within our cohort only 47.1% and 52.9% of patients underwent follow-up ambulatory pH testing and esophageal manometry, respectively. While follow-up tests were scheduled for the remaining patients, all cancelled their appointments for reasons including hospitalization, other illness, and patient request. As testing was not rescheduled in any of these cases, we may reasonably infer that patients for whom follow-up testing was not conducted were doing well, without cause to return for evaluation with what are well-recognized as particularly uncomfortable tests. Our assessment of the efficacy of the LINX device among LTx recipients may therefore be biased in favor of patients who underwent follow-up testing deemed necessary by patient or provider assessment and may not have captured those who achieved entirely favorable results. Further study with rigorous acquisition of follow-up pH testing and esophageal manometry is required to test this hypothesis and further elucidate the efficacy of the LINX device in LTx recipients; in the future, increased utilization of BRAVO pH studies may reduce patient discomfort and increase the proportion of patients who are amenable to follow-up testing. Likewise, a subset of patients in our study who underwent early post-transplant anti-reflux surgery due to the presence of severe GERD on pre-transplant evaluation, did not undergo repeat post-transplant pH testing prior to LINX implantation. As esophageal acid exposure may change considerably after LTx, future studies should ensure that all patients, regardless of pre-transplant GERD severity, undergo repeat post-transplant pH testing prior to anti-reflux surgery to facilitate better understanding of the expected change in esophageal acid exposure time associated with post-LTx LINX implantation. Finally, the short duration of post-LINX implantation follow-up in this study limits our ability to comment on the true safety of the LINX device. While our findings offer important new insight into the potential utility of the LINX device in LTx recipients, demonstrating safety comparable to that observed in the first year after traditional fundoplication in addition to highlighting the potential to hasten postoperative recovery through shorter postoperative hospital stays and prompt return to normal dietary habits, additional studies are needed to assess long-term outcomes of this relatively new technology in chronically immunosuppressed populations. Specifically, while our results suggest that pulmonary function was unchanged across pre- and post-LINX measurements, the short duration of follow-up in this study limits our ability to provide robust examination of the pre- and post-LINX incidence and prevalence of CLAD, the prevention of which remains a leading reason for aggressive surgical management of GERD in LTx recipients. As a sustained decline in FEV1 to ≤80% of baseline for at least 3 weeks is required to diagnose “probable” CLAD, or at least 3 months to diagnose “confirmed” CLAD (4), ongoing follow-up beyond one year is required to better elucidate the trajectory of FEV1 post-LINX implantation and understand the ability of the LINX device to prevent or improve CLAD among treated patients. Likewise, as new techniques for surgical management of post-transplant GERD continue to evolve, future studies should explore the safety and efficacy of the LINX device in comparison to other alternative anti-reflux procedures including the Stretta procedure and transoral incisionless fundoplication.
Conclusions
To our knowledge, this is the first study to examine the use of novel implantable devices for management of post-transplant GERD in LTx recipients. Use of the LINX Reflux Management System in a cohort of LTx recipients at our institution was associated with an overall favorable short-term safety profile compared to traditional fundoplication, and preserved pulmonary function up to one year post-operatively. However, only a small subset of patients achieved reduced or normal esophageal acid exposure times. While our findings suggest that the LINX Reflux Management System is a safe method for surgical management of post-LTx GERD, further investigation is needed to understand its efficacy and long-term safety in this chronically immunosuppressed population.
Acknowledgments
Funding: This work was supported by the National Institutes of Health [TL1TR002555 to SEH and AYC], 5T32HL069749 to OKJ], and Torax Medical, Inc. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, or Torax Medical, Inc.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-3276
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-3276
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-3276). MGH reports grants from Torax, during the conduct of the study. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Duke University (Pro00058718) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Young LR, Hadjiliadis D, Davis RD, et al. Lung Transplantation Exacerbates Gastroesophageal Reflux Disease. Chest 2003;124:1689-93. [Crossref] [PubMed]
- Davis CS, Shankaran V, Kovacs EJ, et al. Gastroesophageal reflux disease after lung transplantation: Pathophysiology and implications for treatment. Surgery 2010;148:737-44; discussion 744-5. [Crossref] [PubMed]
- Hadjiliadis D, Davis RD, Steele MP, et al. Gastroesophageal reflux disease in lung transplant recipients. Clin Transplant 2003;17:363-8. [Crossref] [PubMed]
- Verleden GM, Glanville AR, Lease ED, et al. Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment—A consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transplant 2019;38:493-503. [Crossref] [PubMed]
- Hartwig MG, Appel JZ, Li B, et al. Chronic aspiration of gastric fluid accelerates pulmonary allograft dysfunction in a rat model of lung transplantation. J Thorac Cardiovasc Surg 2006;131:209-17. [Crossref] [PubMed]
- Li B, Hartwig MG, Appel JZ, et al. Chronic aspiration of gastric fluid induces the development of obliterative bronchiolitis in rat lung transplants. Am J Transplant 2008;8:1614-21. [Crossref] [PubMed]
- D’Ovidio F, Mura M, Ridsdale R, et al. The effect of reflux and bile acid aspiration on the lung allograft and its surfactant and innate immunity molecules SP-A and SP-D. Am J Transplant 2006;6:1930-8. [Crossref] [PubMed]
- Fisichella PM, Davis CS, Lundberg PW, et al. The protective role of laparoscopic antireflux surgery against aspiration of pepsin after lung transplantation. Surgery 2011;150:598-606. [Crossref] [PubMed]
- Hartwig MG, Duane Davis R. Gastroesophageal reflux disease-induced aspiration injury following lung transplantation. Curr Opin Organ Transplant 2012;17:474-8. [Crossref] [PubMed]
- Fisichella PM, Davis CS, Kovacs EJ. A review of the role of GERD-induced aspiration after lung transplantation. Surg Endosc 2012;26:1201-4. [Crossref] [PubMed]
- Hartwig MG, Anderson DJ, Onaitis MW, et al. Fundoplication After Lung Transplantation Prevents the Allograft Dysfunction Associated With Reflux. Ann Thorac Surg 2011;92:462-8; discussion 468-9. [Crossref] [PubMed]
- Lau CL, Palmer SM, Howell DN, et al. Laparoscopic antireflux surgery in the lung transplant population. Surg Endosc 2002;16:1674-8. [Crossref] [PubMed]
- Palmer SM, Miralles AP, Howell DN, et al. Gastroesophageal reflux as a reversible cause of allograft dysfunction after lung transplantation. Chest 2000;118:1214-7. [Crossref] [PubMed]
- Davis RD, Lau CL, Eubanks S, et al. Improved lung allograft function after fundoplication in patients with gastroesophageal reflux disease undergoing lung transplantation. J Thorac Cardiovasc Surg 2003;125:533-42. [Crossref] [PubMed]
- Robertson AGN, Krishnan A, Ward C, et al. Anti-reflux surgery in lung transplant recipients: Outcomes and effects on quality of life. Eur Respir J 2012;39:691-7. [Crossref] [PubMed]
- Bonavina L, Demeester T, Fockens P, et al. Laparoscopic sphincter augmentation device eliminates reflux symptoms and normalizes esophageal acid exposure: One-and 2-year results of a feasibility trial. Ann Surg 2010;252:857-62. [Crossref] [PubMed]
- Du X, Hu Z, Yan C, et al. A meta-analysis of long follow-up outcomes of laparoscopic Nissen (total) versus Toupet (270°) fundoplication for gastro-esophageal reflux disease based on randomized controlled trials in adults. BMC Gastroenterol 2016;16:88. [Crossref] [PubMed]
- Lipham JC, DeMeester TR, Ganz RA, et al. The LINX ® reflux management system: Confirmed safety and efficacy now at 4 years. Surg Endosc 2012;26:2944-9. [Crossref] [PubMed]
- Bonavina L, Saino G, Bona D, et al. One hundred consecutive patients treated with magnetic sphincter augmentation for gastroesophageal reflux disease: 6 years of clinical experience from a single center. J Am Coll Surg 2013;217:577-85. [Crossref] [PubMed]
- Asti E, Bonitta G, Lovece A, et al. Longitudinal comparison of quality of life in patients undergoing laparoscopic Toupet fundoplication versus magnetic sphincter augmentation: Observational cohort study with propensity score analysis. Medicine (Baltimore) 2016;95:e4366 [Crossref] [PubMed]
- Palmer SM, Alexander BD, Sanders LL, et al. Significance of blood stream infection after lung transplantation: analysis in 176 consecutive patients. Transplantation 2000;69:2360-6. [Crossref] [PubMed]
- Ganz RA, Edmundowicz SA, Taiganides PA, et al. Long-term Outcomes of Patients Receiving a Magnetic Sphincter Augmentation Device for Gastroesophageal Reflux. Clin Gastroenterol Hepatol 2016;14:671-7. [Crossref] [PubMed]
- Bonavina L, Saino GI, Bona D, et al. Magnetic augmentation of the lower esophageal sphincter: Results of a feasibility clinical trial. J Gastrointest Surg 2008;12:2133-40. [Crossref] [PubMed]
- Ganz RA, Peters JH, Horgan S, et al. Esophageal sphincter device for gastroesophageal reflux disease. N Engl J Med 2013;368:719-27. [Crossref] [PubMed]
- Hoppo T, Jarido V, Pennathur A, et al. Antireflux surgery preserves lung function in patients with gastroesophageal reflux disease and end-stage lung disease before and after lung transplantation. Arch Surg 2011;146:1041-7. [Crossref] [PubMed]
- Humphries LA, Hernandez JM, Clark W, et al. Causes of dissatisfaction after laparoscopic fundoplication: The impact of new symptoms, recurrent symptoms, and the patient experience. Surg Endosc 2013;27:1537-45. [Crossref] [PubMed]


