Bronchial artery embolization for malignant hemoptysis: a single institutional experience
Introduction
Massive hemoptysis is an emergent condition with a high associated mortality of 50-100% when treated conservatively (1-3). Life-threatening hemoptysis represents a late, often deadly endpoint of a variety of underlying lung pathologies. Lung malignancy accounts for an estimated 30% of hemoptysis, with approximately 10% of lung cancer patients developing massive hemoptysis (4,5). Bronchial artery embolization (BAE) has become a well-established treatment option in the management of life-threatening hemoptysis (6-16). However, malignancy-related hemoptysis from primary or metastatic lung cancer has received less attention in the BAE literature than other benign, more common causes of hemoptysis. Fartoukh et al. reported malignancy as an independent predictor of in-hospital mortality in patients presenting with hemoptysis, carrying over a 7-fold higher risk of death than hemoptysis from other causes (2). Furthermore, the published data suggest poor outcomes in patients undergoing BAE for malignant hemoptysis (6,17,18). The aim of this study was to report our experience with BAE in treating patients with a known underlying lung malignancy and characterize clinical outcomes to identify potential risk factors predictive of short-term mortality.
Materials and methods
Study population
Following approval from our Institutional Review Board and Clinical Trials Review Committee, we retrospectively reviewed the clinical records of 26 consecutive patients with hemoptysis and lung malignancy undergoing BAE between 2003 and 2013. Indications for embolization included moderate to massive hemoptysis, defined as at least 50 mL of expectorated blood within 24 hours, or small volume hemoptysis refractory to conservative management and/or flexible bronchoscopy. Decision to pursue BAE was made by the primary clinical service (pulmonology or thoracic surgery) in a coordinated interdisciplinary fashion between the radiation oncology and interventional radiology teams. Outcomes were investigated retrospectively from integrated inpatient and outpatient medical records. The data categorized in Tables 1-3 were collected from the electronic medical record of each patient.
Full table
Full table
Full table
Procedural technique
All patients provided written informed consent for the procedure. Platelet count, international normalized ratio, and activated partial thomboplastin time were collected and any pre-existing coagulopathies corrected. Pre-procedural imaging studies were reviewed when available.
Arterial access into the right or left common femoral artery was obtained using a micropuncture set (Cook, Bloomington, IN, USA). A 5-French vascular sheath was then placed. A 5-French multi side hole catheter was advanced into the thoracic aorta over a 0.035 inch J wire (Cook, Bloomington, IN, USA) to obtain a thoracic aortogram. Any hypertrophied bronchial or parasitized systemic arteries from the aorta or thyrocervical trunk identified on pre-procedural imaging were targeted for embolization. Subsequent selected bronchial arteries were catheterized using a 5-French diagnostic catheter and microwire/microcatheter system (Transcend and Renegade Hi-Flo; Boston Scientific, Natick, MA, USA). Embolization was performed when localizing findings were present, that is, when active extravasation or suspicious tumor neovascularity or hypervascularity was documented; in one case there were no positive angiographic findings though a vessel coursing in the location of the known bleeding tumor was treated. In cases of no significant bronchial artery angiographic findings to explain the clinical presentation and in cases of repeat exam for clinical failure, selective IMA catheterization was employed to confirm absence of collateral supply. The angiographic endpoint for these cases included embolic arrest of any active extravasation, stasis or near-stasis of embolized vessel(s), or non-visualization of parenchymal tumor blush (n=2, 20 and 4, respectively). Embolic materials used included a single agent or combination of trisacryl gelatin microspheres (Embospheres; Merit Medical Systems, South Jordan, UT, USA) of 500-700 or 700-900 um sizes, coils (Interlock; Boston Scientific, Natick, MA, USA), or Gelfoam (Pfizer, New York, NY, USA). Coils were deployed only in cases of significant arterio-venous shunting (n=4) to prevent non-target embolization.
Definitions
Definitions of severe, moderate, and small volume of hemoptysis were a documented or estimated volume of >300 cc (n=6), 50-300 cc (n=15), and <50 cc (n=5) in the 24 hours preceding admission, respectively (2,19-21). Criteria for positive bronchoscopy for active bleeding included documented visualization of active bleeding, oozing, or fresh blood. Thrombocytopenia was considered to be significant if platelet count was less than 100,000/mcL at time of presentation. In cases without prior cross sectional imaging, diagnostic arteriography was initially performed with an aortogram followed by selective arteriograms of any hypertrophied vessels. Angiography positive for bleeding was defined as active extravasation on DSA and for tumor was findings strongly suggestive of tumor blush, tumoral vascularity, parasitized vessels, or significantly hypertrophied arteries in expected location of tumor. History of COPD was based on chart documentation, PFTs, or moderate to severe emphysematous changes on CT. Hemodynamic instability was defined as hypotension (systolic blood pressure <90 mmHg) or requirement of vasopressors upon or after presentation. Comorbidities qualifying as significant included coronary artery disease, arrhythmia, valvular heart disease, hypertension, hyperlipidemia, diabetes mellitus, chronic renal failure, chronic obstructive pulmonary disease, obstructive sleep apnea, recent venous thromboembolic disease, inflammatory bowel disease, multiple sclerosis, and history of unrelated malignancy.
Endpoints
Endpoints included clinical success (defined by complete or near-complete resolution of hemoptysis within 48 hours), recurrence of hemoptysis (any clinically significant increase in hemoptysis following initial clinical success), in-hospital mortality, and 6-month mortality.
Statistics
Statistical analysis was performed to compare the subset of patients surviving 6 months beyond BAE to those that did not using Chi-square analysis for discrete variables and t-test for continuous variables. Multi-variate analysis was also performed to assess for factors predicative of clinical success or failure. All statistical analysis was undertaken using the STATA software package (Version 12.0, 1985-2011; StataCorp LP, College Station, TX, USA).
Results
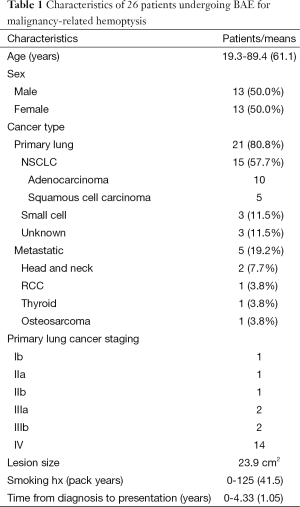
Twenty-six patients were referred to interventional radiology for BAE for hemoptysis from lung malignancy during the study period, 13 (50.0%) male and 13 (50.0%) female. Primary lung cancer accounted for malignant lesions in 21 (80.8%) patients, with the remaining five (19.2%) representing metastatic pulmonary disease. Histology of patients with primary lung cancer were non-small-cell carcinoma in 15 patients (57.7%) (which included 10 adenocarcinoma, five squamous cell carcinoma), small cell carcinoma in three patients (11.5%), and was unknown in three patients (11.5%). Among patients with primary lung tumors, 18 of 21 had unresectable stage III or IV disease at time of presentation.
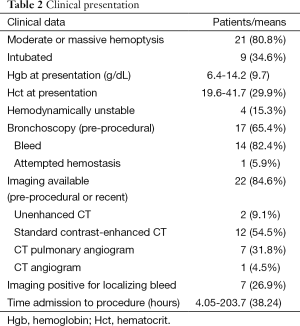
Moderate or massive hemoptysis was observed in 21 (80.8%) of patients. Nine (34.6%) of patients were intubated prior to undergoing BAE either for respiratory failure or airway protection. Four (15.3%) were hemodynamically unstable at presentation. Pre-procedural flexible bronchoscopy was performed on 17 patients (65.4%), which revealed an active bleed in 14 (82.4%), of which only one patient (5.9%) was attempted to be treated bronchoscopically. No bronchoscopic complications such as induced bleeding were reported. The mean lesion dimensions were 5.7×4.2 cm2 (axial area 23.9 cm2). Average time from diagnosis of lung malignancy was 1.05±1.28 years. All patients had a history chemotherapy and/or radiation therapy to the lungs; only four patients were currently undergoing radiation therapy and two undergoing chemotherapy at the time of presentation.
Preprocedural (within 3 days) or recent (within 14 days) cross sectional imaging was available in 22 of 26 patients. This included non-enhanced CT in two patients, standard contrast-enhanced CT in 12 patients, CT pulmonary angiogram in seven patients, and CT angiogram in one patient. Active hemorrhage was visualized on seven exams (31.8%). Vascular encasement, attenuation, or occlusion by mass lesion was identified on seven exams (31.8%).
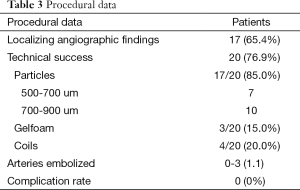
Embolization was performed by seven different operators during the study period with an average of 8.16±4.98 years of experience at the time of procedure. On arteriography, 17 (65.4%) demonstrated localizing findings. Technical success was achieved in 20 patients (76.9%). The six technical failures included one or a combination of the following factors: patient cooperation (n=1), concern for non-target embolization due to reflux of contrast into the left subclavian artery (n=1), failure to identify or select bronchial arteries (n=4), and no localizing findings on angiography, as defined above (n=3). The number of vessels embolized was one in 14 patients, two in four patients, and three in two patients. A total of 17 arteries were embolized using trysacryl gelatin microsphere particles (seven with 500-700 um particles and ten with 700-900 um particles), three arteries with Gelfoam, and four arteries using coils. The observed complication rate using Society of Interventional Radiology (SIR) definitions was 0% (10). Only two patients with negative catheter angiograms received post-procedural CTA, neither of which yielded localizing findings.
Clinical success was achieved in 15 of the 20 patients among the technically successful BAE procedures (75.0%). Recurrence was observed in three patients (15.0%), one of which required repeat BAE (two resolved spontaneously upon readmission in one patient and transfer to ICU in the other). One patient with Stage IIB disease was initially deemed a high risk surgical candidate by the thoracic surgery service due to respiratory difficulty from ongoing moderate-volume hemoptysis as well as history of coronary artery disease, COPD, CVA, and PE; the patient was discharged from the hospital after stabilization and underwent extrapleural pneumonectomy after medical optimization. One of the five patients who by definition did not achieve clinical success was determined to have additional pharyngeal bleeding within 48 hours; laryngoscopy showed likely source as friable tissue from radiation therapy for prior laryngeal cancer, resulting in multiple embolizations of external carotid artery branches by the neurointerventional service, though this patient eventually required two surgical interventions by the ENT service for definitive treatment. Three patients received palliative radiation therapy following BAE.
Sixteen of 26 patients (61.5%) survived less than 6 months after presentation, with an in-hospital mortality observed in five (19.2%) (Figures 1,2). In-hospital deaths were attributed to respiratory failure from continued hemoptysis in four patients and arrhythmia in one patient. The majority of patients that died after discharge but within 6 months had been discharged to hospice. Statistically significant predictors of 6-month mortality were intubation status, hemoglobin and hematocrit at presentation, and significant thrombocytopenia (Table 4).
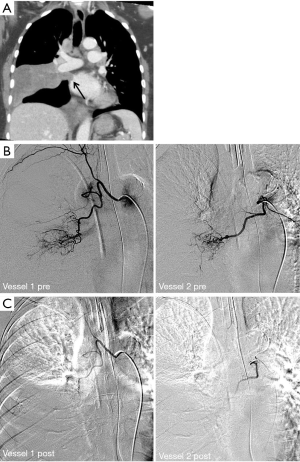
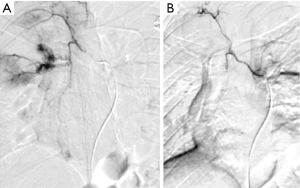

Full table
Discussion
First performed in 1973 by Remy et al., BAE has become a widely accepted treatment option in the management of patients with hemoptysis. Since then, there has been little published regarding BAE specifically for malignancy-related hemoptysis. Hemoptysis occurs by similar mechanisms in benign and malignant etiologies, that is, by local necrosis and inflammation of blood vessels (22). Several studies have suggested poorer outcomes in oncology patients presenting with hemoptysis, even in those with low acuity presentations, likely secondary to comorbidities (22). The current study comprises a large analysis of outcomes in patients with malignant-hemoptysis undergoing BAE.
The pathophysiology of hemoptysis in malignancy stems from localized hypoxia as well as direct expression of angiogenic growth factors by neoplastic cells, which induce neovascularity from bronchial arteries as well as recruitment of systemic vasculature, especially when in close proximity to pleural surfaces. Collateral vascular walls are thin and friable which can bleed, or even may lead to pseudoaneurysm formation and rupture (22,23).
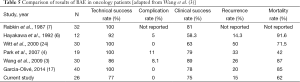
Our data support BAE as an effective treatment for hemoptysis secondary to lung malignancy, with a favorable safety profile. Our clinical success rate of 75.0% is comparable to the 58-89% reported by other groups when accounting for considerable variety in definitions and differences in practice (4,6,7,17,24). Our technical success rate 76.9% is lower than that seen in most other studies on BAE from all causes (7-9,11,12), and specifically of those in oncology patients, which ranges between 86-100% (4,6,7,17,24). Finally, our recurrence rate along with that reported by Hayakawa et al. are amongst the lowest reported in the literature (3-6,9,12). A summary of the findings in the other oncology cohorts and ours comprises Table 5.
Despite the demonstrated clinical effectiveness of BAE, our data suggest that oncology patients still experience a high mortality rate from their underlying disease. In-hospital mortality was observed in nearly one-fifth of all patients. We show intubation status, hemoglobin and hematocrit at presentation, and marked thrombocytopenia to be statistically significant prognostic factors affecting survival in patients receiving BAE. Factors that notably were not predictive of mortality included age, gender, primary versus metastatic disease, degree of hemoptysis, and hemodynamic instability. Particularly interesting to note is that patients with severe hemoptysis were no more likely to expire within 6 months than those with small volume hemoptysis. In support of these findings, Park et al. showed a mortality rate of 42% in patients, most of whom they describe as presenting with only trivial hemoptysis (4); in contrast, over 80% of our patients presented with moderate or severe hemoptysis, despite a similar mortality rate.
Implications of our data and the few similar studies in the literature are important for clinicians caring for oncology patients presenting with hemoptysis. A high expected mortality despite treatment with BAE may be especially pertinent in patients with less acute presentations, in which the long-term mortality may carry more weight in clinical decision making than in cases of acute, massive life-threatening hemoptysis. Apart from its utility in emergent cases to avoid immediate death by asphyxiation, our observations support a more selective approach in utilizing BAE in oncology patients, particularly in those without severe and imminently fatal co-morbidities. In addition, regardless of presentation, these results carry relevance in goals-of-care discussions with patients and their families upon presentation, and in end-of-life planning even prior to onset of hemoptysis.
Limitations of this study include its retrospective nature and sample size, despite being a relatively large cohort for oncology patients, as stated above. Any retrospective analysis of a condition with multidisciplinary management options will contain bias based on the choices and failures of initial attempted interventions prior to the procedure being examined. It is unknown in our study, for example, the number of patients presenting with hemoptysis treated effectively with bronchoscopy, yielding more retractable and possibly poorer condition of patients presenting to interventional radiology. Other confounding variables include inhomogeneity of embolic agents, which could potentially affect the degree of hemostasis due to differences in extent of tissue necrosis. In addition, a low technical success rate confounds and artificially elevates our clinical success rate, which excludes cases of technical failure. Also, while the scope of our objective was intentionally narrow, comparison with patients presenting with hemoptysis from benign etiologies would lend a clearer perspective for the clinician on the utility of the present results. This is a promising direction of future investigation. The retrospective nature of the study also particularly limits evaluation of cause of death, which in most patients is further confounded by end-of-life, hospice, and palliative care management options undertaken. Further work would ideally include a prospective study to validate our observations, which could theoretically also make possible the distinction between mortality from bleeding and that from other causes, particularly regarding those deaths that occurred outside of the hospital. And finally, a recent well-conducted prospective study regarding segmental pulmonary arterial chemoembolization in combination with radiofrequency ablation has earned significant attention for treatment of unresectable lung malignancies (25). We feel the utilization of these modalities in the management of patients presenting with acute hemoptysis warrants further investigation.
In conclusion, BAE is a safe and effective treatment in malignancy-related hemoptysis. However, patients experience a high in-hospital and 6-month mortality despite clinical success of the procedure from their underlying disease. Some clinical prognostic factors upon presentation may include intubation status, low hemoglobin/hematocrit and thrombocytopenia. Data regarding mortality in oncology patients receiving BAE may have strong implications in clinical decision-making, referral for BAE, and in palliative care discussions.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chun JY, Belli AM. Immediate and long-term outcomes of bronchial and non-bronchial systemic artery embolisation for the management of haemoptysis. Eur Radiol 2010;20:558-65. [PubMed]
- Fartoukh M, Khoshnood B, Parrot A, et al. Early prediction of in-hospital mortality of patients with hemoptysis: an approach to defining severe hemoptysis. Respiration 2012;83:106-14. [PubMed]
- Wang GR, Ensor JE, Gupta S, et al. Bronchial artery embolization for the management of hemoptysis in oncology patients: utility and prognostic factors. J Vasc Interv Radiol 2009;20:722-9. [PubMed]
- Park HS, Kim YI, Kim HY, et al. Bronchial artery and systemic artery embolization in the management of primary lung cancer patients with hemoptysis. Cardiovasc Intervent Radiol 2007;30:638-43. [PubMed]
- McGuinness G, Beacher JR, Harkin TJ, et al. Hemoptysis: prospective high-resolution CT/bronchoscopic correlation. Chest 1994;105:1155-62. [PubMed]
- Hayakawa K, Tanaka F, Torizuka T, et al. Bronchial artery embolization for hemoptysis: immediate and long-term results. Cardiovasc Intervent Radiol 1992;15:154-8; discussion 158-9. [PubMed]
- Rabkin JE, Astafjev VI, Gothman LN, et al. Transcatheter embolization in the management of pulmonary hemorrhage. Radiology 1987;163:361-5. [PubMed]
- Rémy J, Voisin C, Dupuis C, et al. Treatment of hemoptysis by embolization of the systemic circulation. Ann Radiol (Paris) 1974;17:5-16. [PubMed]
- Uflacker R, Kaemmerer A, Neves C, et al. Management of massive hemoptysis by bronchial artery embolization. Radiology 1983;146:627-34. [PubMed]
- Brinson GM, Noone PG, Mauro MA, et al. Bronchial artery embolization for the treatment of hemoptysis in patients with cystic fibrosis. Am J Respir Crit Care Med 1998;157:1951-8. [PubMed]
- Mal H, Rullon I, Mellot F, et al. Immediate and long-term results of bronchial artery embolization for life-threatening hemoptysis. Chest 1999;115:996-1001. [PubMed]
- Ramakantan R, Bandekar VG, Gandhi MS, et al. Massive hemoptysis due to pulmonary tuberculosis: control with bronchial artery embolization. Radiology 1996;200:691-4. [PubMed]
- Swanson KL, Johnson CM, Prakash UB, et al. Bronchial artery embolization: experience with 54 patients. Chest 2002;121:789-95. [PubMed]
- Fernando HC, Stein M, Benfield JR, et al. Role of bronchial artery embolization in the management of hemoptysis. Arch Surg 1998;133:862-6. [PubMed]
- Knott-Craig CJ, Oostuizen JG, Rossouw G, et al. Management and prognosis of massive hemoptysis. Recent experience with 120 patients. J Thorac Cardiovasc Surg 1993;105:394-7. [PubMed]
- Yu-Tang Goh P, Lin M, Teo N, et al. Embolization for hemoptysis: a six -year review. Cardiovasc Intervent Radiol 2002;25:17-25. [PubMed]
- Garcia-Olivé I, Sanz-Santos J, Centeno C, et al. Results of bronchial artery embolization for the treatment of hemoptysis caused by neoplasm. J Vasc Interv Radiol 2014;25:221-8. [PubMed]
- Katoh O, Kishikawa T, Yamada H, et al. Recurrent bleeding after arterial embolization in patients with hemoptysis. Chest 1990;97:541-6. [PubMed]
- Yoon W, Kim JK, Kim YH, et al. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics 2002;22:1395-409. [PubMed]
- Marshall TJ, Jackson JE. Vascular intervention in the thorax: bronchial artery embolization for haemoptysis. Eur Radiol 1997;7:1221-7. [PubMed]
- Najarian KE, Morris CS. Arterial embolization in the chest. J Thorac Imaging 1998;13:93-104. [PubMed]
- Winter SM, Ingbar DH. Massive hemoptysis: Pathogenesis and management. J Intensive Care Med 1988;3:171-88.
- Sopko DR, Smith TP. Bronchial artery embolization for hemoptysis. Semin Intervent Radiol 2011;28:48-62. [PubMed]
- Witt Ch, Schmidt B, Geisler A, et al. Value of bronchial artery embolisation with platinum coils in tumorous pulmonary bleeding. Eur J Cancer 2000;36:1949-54. [PubMed]
- Gadaleta CD, Solbiati L, Mattioli V, et al. Unresectable lung malignancy: combination therapy with segmental pulmonary arterial chemoembolization with drug-eluting microspheres and radiofrequency ablation in 17 patients. Radiology 2013;267:627-37. [PubMed]