Effect of dobutamine on lung aquaporin 5 in endotoxine shock-induced acute lung injury rabbit
Introduction
Acute lung injury (ALI) remains a major contributor to morbidity and mortality in critically ill patients. The frequency of ALI in the United States alone is ~190,000 cases/year with a result of 74,500 deaths (1). Unfortunately, the mortality remains at a high level in patients with ALI, despite that many studies aimed at investigating the mechanism of ALI have been performed. ALI is characterized by alveolar epithelial injury leading to increased permeability of the alveolar-capillary barrier, which often manifests with increased extravascular lung water and the formation of pulmonary edema. Recently, extravascular lung water, which is closely related to the formation of pulmonary edema, was shown to be an independent predictor of intensive care unit mortality in patients with septic shock (2). Accordingly, understanding the mechanisms of water transport across biological membranes and alleviating pulmonary edema during severe sepsis and septic shock may reverse the progression of lung injury.
The aquaporins (AQPs) are a family of small integral membrane proteins which are expressed in both prokaryotes and eukaryotes. Among the 13 isoforms in the mammalian AQP family identified to date (3), aquaporin 5 (AQP5) is a water-selective AQP which is widely distributed in various tissues throughout the body such as granules of Brunner glands in the duodenum (4), salivary gland (5), cornea (6), lens (7) and apical membrane of type I alveolar epithelial cells (8,9). AQP5 has been thought to be a pivotal participant in the fluid secretion of the submucosal serous gland. Studies in animal’s models have shown that AQP5 plays a key role in maintaining normal lung water homeostasis and changes in the amount of cell surface expression of AQP5 may contribute to abnormal water metabolism in various lung diseases (10). A study examining the distribution of AQP5 in lacrimal gland showed that a selective defect in the trafficking of AQP5 in the lacrimal gland may be responsible for the pathogenesis of Sjogren syndrome (11). Furthermore, targeted deletion of AQP5 in mice leads to significantly reduction of fluid secretion from submucosal glands (12) and a 10-fold decrease in osmotic water permeability of the alveolar-capillary barrier in distal lungs (13). These previous studies have showed that AQP5 plays a pivotal role in maintaining normal tissues water homeostasis, and abnormal expression and/or dysfunction of AQP5 may contribute to abnormal water metabolism in lung, resulting in the formation of pulmonary edema.
β-adrenergic receptors (β-ARs) are prototypic members of the G-protein-coupled receptors which participate in regulating the concentration of intracellular second messengers such as cyclic AMP (cAMP) (14). β-receptor binding activates trimeric G proteins, leading to the information of stimulatory G proteins (15). Stimulatory G proteins then activate adenyl cyclase, resulting in an increase in intracellular cAMP concentration, which subsequently leads to protein kinase A formation. Protein kinase A directly phosphorylates the specific sequence of AQP5 to regulate its activity, which subsequently increase membrane permeability to water (15,16).
Dobutamine, as a synthetic β-AR agonist, is commonly administered to patients with low-output states secondary to ALI/ARDS to maintain tissue perfusion. In addition, dobutamine is usually used in patients with non-cardiac pulmonary edema and investigations have demonstrated that dobutamine has protective effect on ALI (17,18). However, the underlying mechanism is unclear. In the present study, we focused on the effect of dobutamine on AQP5 expression in the lungs of New Zealand rabbits with ALI induced by endotoxin shock and further addressing the possible mechanism.
Materials and methods
Experimental animal
Seventy male New Zealand white rabbits, weighing approximately 2.0-2.5 kg, were purchased from the Laboratory Animal Center of Nanjing Hospital, Nanjing Medical University (Nanjing, China). The rabbits were housed in standard conditions with food and water ad libitum. All animal studies were approved by the Institutional Animal Use and Care Committee of Nanjing Hospital affiliated to Nanjing Medical University, and the care and handling of the animals were in accordance with the National Institutes of Health guidelines.
Experimental protocols
Rabbits were fasted for 12 h but allowed free access to water before the experiments. Animals were anesthetized with 20% urethane (5 mL/kg). Urethane was given through ear vein. The left carotid artery was cannulated to measure mean arterial pressure (MAP) continuously by using a Spacelab monitor instruments (USA). Left femoral vein was cannulated for medications and fluids. The animals were placed on a heating pad so that the body temperature could be kept at 37.8-40.2 °C. Lactated Ringer’s solution was intravenously administered at 12.5 mL/kg/h by a LC-10ATvp plus Liquid Pump (Shimadzu, Japan).
Rabbits were randomly divided into seven groups (n=10 for each group): sham group, ALI group, dobutamine low-dose group [group ALI + Dob (L)], dobutamine medium-dose group [group ALI + Dob (M)], dobutamine high-dose group [group ALI + Dob (H)], ALI + Dob (H) + ICI (ICI 118,551, a specific beta-2 antagonist, Research Biochemicals International, Natick, MA) group and sham + ICI group. The rabbits in group ALI, group ALI + Dob (L), group ALI + Dob (M), group ALI + Dob (H) and group ALI + Dob (H) + ICI received intravenous LPS (600 μg/kg, Escherichia coli-serotype O55:B5, Sigma, USA) to induce endotoxic shock. Rabbits in group sham and sham + ICI received intravenous normal saline. Two hours after administration of LPS or normal saline, rabbits in group ALI, ALI + Dob (L), ALI + Dob (M), ALI + Dob (H) and ALI + Dob (H) + ICI were immediately resuscitated with Lactated Ringer’s solution intravenously at 12.5 mL/kg/h. Rabbits in group ALI + Dob (L), ALI + Dob (M), ALI + Dob (H) continuously received dobutamine at 2.5, 5, 10 μg/kg/min, respectively, by a Liquid Pump (Shimadzu, Japan). Meanwhile, groups sham + ICI and ALI + Dob (H) + ICI were administrated with ICI 118,551. Ten rabbits from each group were sacrificed 3 h after septic shock respectively. ALI induced by endotoxic shock was confirmed by a lowered MAP (<60% of the baseline).
Sampling and storage
At the end of the experiment, rabbits were sacrificed and quickly perfused with phosphate-buffered saline (PBS) through the right ventricle after extracting the carotid artery blood for arterial blood gas analysis (BWD3-GEM Premier 3,000). And then lungs were removed for tissue analysis.
Measurement of cAMP level in lung
Lung tissue was treated with isobutyryl methylxanthine (Sigma-Aldrich) to inhibit phosphodiesterases. The amount of cAMP in lung tissue was evaluated using a commercial cAMP EIA kit (NewEast Biosciences; Malvern, PA, USA) according to the instructions of the manufacturer.
Wet to dry weight (W/D) ratio of lung
Left lung tissues were weighed and dried in an oven at 70 °C for 48 h to obtain pulmonary W/D ratio.
Pathological examination
Lung tissues were fixed by immersing into 4% paraformaldehyde and routinely processed into paraffin sections (5 μm). These sections were stained with hematoxylin and eosin. Six slices were selected from each group and six fields of each slice were visualized by light microscopy (×400) (Olympus, DP73). The degree of pathological injury was scored based on the following variables: hemorrhage, lung edema, inflammatory cell infiltration, hyaline membrane and atelectasis; The degree of each abnormality was graded numerically from 0 (normal) to 4 (diffuse injury) according to the following criteria (19): no injury =0; injury to 25% of the field =1; injury to 50% of the field =2; injury to 75% of the field =3; diffuse injury =4. Blind analysis was performed to determine the lesion degree of all samples.
Transmission electron microscope
Lung tissue samples were fixed with 2.5% glutaraldehyde in 0.1 M Sorensen’s phosphate buffer (PH 7.2) followed by 1% osmium tetroxide in the same buffer. The specimens were dehydrated through an upgraded acetone series at room temperature. Counterstaining was done in a saturated solution of uranyl acetate followed by lead citrate. Sections were examined with a transmissions electron microscope (×50,000) (Phillips, Netherlands).
Immunohistochemistry
Lung tissue samples were fixed in 4% paraformaldehyde and cut into 5 μm sections. Immunohistochemistry was performed after blocking endogenous peroxidase activity with 3% H2O2 and methanol for 10 min, and non-specific protein binding with 10% sheep serum for 15 min. Sections were then incubated with anti-AQP5 antibody (1:200 dilution; SantaCruz Biotechnology, CA, USA) overnight at 4 °C. After three rinses of 5 min with PBS, sections were incubated with peroxidase-conjugated IgG antibody. Following three rinses of 5 min with PBS, the slides were then stained utilizing the biotin-avidin peroxidase method. After development, slides were counterstained with hematoxylin. The immunoreaction was evaluated with the Imagepro-Plus 12.0 which had been programmed to determine the mean optical density (MOD) of positive areas.
Western blotting analysis
Samples were homogenized in cold RIPA lysis buffer (150 mM NaCl, 25 mM Trise-HCl PH 7.6, 1% NP-40, 1% sodium deoxycholate, 1% SDS, and 1% protease inhibitor cocktail) and lysed 30 min on ice. Total lysate proteins were resolved using 15% SDS-PAGE and transferred to a PVDF-membrane. Membrane was blocked with blocking solution (5% nonfat dry milk in TBS-T) at room temperature for 2 h and then incubated overnight at 4 °C with anti-AQP5 polyclonal antibody (1:400, SantaCruz biotechnology, CA, USA). After washing in TBS-T, the membrane was incubated with goat anti-rabbit IgG conjugated to horseradish peroxidase at room temperature for 2 h, then washed in TBS-T. Immunoblots were visualized on photographic films using Enhanced Chemical Luminescence reagents. Bands were quantified using Gel-Pro32 software. The relative expression of AQP5 was normalized to that of GAPDH.
Statistical analysis
Data are expressed as mean ± SD. Statistical analysis was performed using SPSS 17.0 statistic software (SPSS Inc., USA). The differences among the different groups were analyzed by one-way ANOVA (LSD t-test). P<0.05 was considered statistically significant.
Results
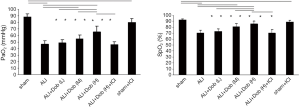
Changes of PaO2 and SpO2 level in different groups
As demonstrated in Figure 1A,B, LPS produced significant decrease of PaO2 and SpO2 in group ALI compared with group sham (P<0.05). PaO2 and SpO2 in group ALI + Dob (M) and ALI + Dob (H) were significantly improved by dobutamine when compared with group ALI (P<0.05). Compared with group ALI + Dob (L), SpO2 in group ALI + Dob (M) and ALI + Dob (H) was also increased (P<0.05) and PO2 in group ALI + Dob (H) increased significantly (P<0.05). However, SpO2 and PaO2 in group ALI + Dob (H) + ICI lowered obviously than that in group ALI + Dob (H).
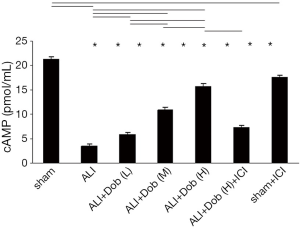
The cAMP concentration of lung tissue in different groups
As shown in Figure 2, the cAMP concentration was markedly lower at 3 h (P<0.01) in group ALI than that in group sham and it was up-regulated in a dose-dependent manner by dobutamine treatment in group ALI + Dob (L). ALI + Dob (M) and ALI + Dob (H). But the level of cAMP in group ALI + Dob (H) + ICI was decreased that that in group ALI + Dob (H) (P<0.05), and it was lessened in group sham + ICI compared with that in group sham (P<0.05).
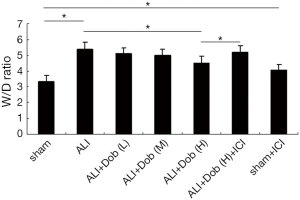
Changes of lung tissue W/D ratio in different groups
Figure 3 showed that the W/D ratio was significantly higher in group ALI than that in group sham at 3 h after shock (P<0.01). Compared with group ALI, the W/D ratio in group ALI + Dob (L) and ALI + Dob (M) did not have statistically significant differences (P>0.05), while the W/D ratio in group ALI + Dob (H) was decreased obviously (P<0.01); additionally, the W/D ratio in group ALI + Dob (H) + ICI was higher than that in group ALI + Dob (H) and it was the same in group sham + ICI than that in group sham.
The lung histological alteration in different groups
Figure 4 demonstrated the results of lung tissue pathological examination. (A) Lungs were collected 3 h after shock and subject to histological staining; (B) the lung injury scores in different groups.
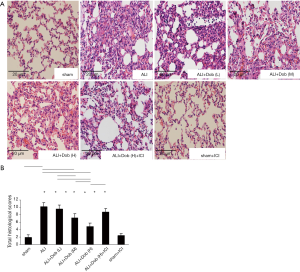
As demonstrated in Figure 4A, only few inflammatory cells were observed in group sham, however, the lung injury in group ALI was very serious after LPS treatment. After administration of dobutamine, lung injury had been improved in different degrees. Interestingly, the lung damage had not been significantly alleviated in group ALI + Dob (H) + ICI compared with that even in group ALI + Dob (L). In addition, lung histological alteration in group sham + ICI was nearly the same as that in group sham. Figure 4B showed that the histological scores were significantly higher in group ALI than that in group sham (P<0.01). Administration of dobutamine reversed the lung injury in a dose-dependent manner as indicated by decreasing histological scores of lung tissues in different groups. Nevertheless, the total histological scores in group ALI + Dob (H) + ICI were increased significantly compared with that in group ALI + Dob (H) when administration of ICI 188,551, a specific beta-2 antagonist.
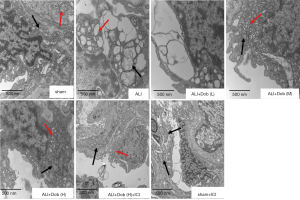
The lung ultrastructural alteration in different groups
As shown in Figure 5, group sham revealed that alveolar type I epithelial cells (AT I) had only mild injury. However, group ALI exhibited that mitochondria were swelled, most of mitochondrial cristae were broke off or even disappeared in vacuoles degeneration; endoplasmic reticulum expanded significantly, forming different sizes vacuoles degeneration. Dobutamine administrated in group ALI + Dob (L) showed no significant effect on the improvement of AT I, but ultrastructural injury of AT I in group ALI + Dob (M) and ALI + Dob (H) improved obviously. However, the degree of ultrastructural lesion in group ALI + Dob (H) + ICI did not get significantly improved compared with that in group ALI + Dob (H).
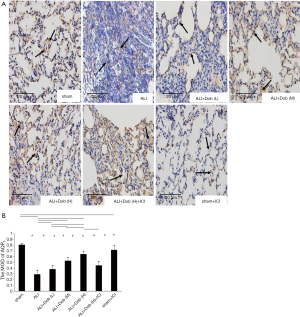
The distribution and expression of lung tissue AQP5 protein in different groups
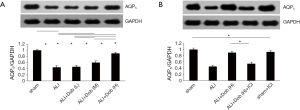
Immunohistochemistry demonstrated AQP5 was located in the cell membrane (indicated by black arrows, Figure 6A). As seen in Figure 6B, group ALI revealed a significant decrease in the MOD of the immunohistochemically positive areas of AQP5 (P<0.05) compared with group sham and it was up-regulated by dobutamine in a dose-dependent manner. The results were further confirmed by Western blot (Figure 7A,B). Nevertheless, the distribution and expression of AQP5 protein decreased obviously in group ALI + Dob (H) + ICI compared with that in group ALI + Dob (H) when effected by ICI 118,551 (P<0.05).
Discussion
LPS is a glycolipid which is the major constituent of the outermost membrane of gram-negative bacteria (20) and it has been thought to be responsible for ALI/ARDS. Previous ALI models have been established by intratracheal/intranasal LPS instillation (21,22). However, local LPS exposure in lungs may not lead to systemic inflammation and multi-organ failure (23). Accordingly, we established lung injury model induced by endotoxic shock by injection of LPS intravenously and studied the protective effect of dobutamine. Microscopy studies of lung tissue in group ALI showed typical ALI. Dobutamine could mitigate ALI in a dose-dependent manner as indicated by histological and ultrastructural studies and decreased pulmonary edema as well as the arterial blood gas analysis. In addition, the result of inflammatory cells in lung tissue decreased after the treatment of dobutamine further supported that β-AR agonist had significant anti-inflammatory effect (15,24).
In the present study, pulmonary edema was detected by W/D ratio analyses in rabbits’ lungs after administration of LPS. We found that endotoxin shock-induced ALI led to a significant increased W/D ratio compared with that of control group and the increase in W/D ratio was highly consistent with the presence of pulmonary edema. In addition, the decreased AQP5 protein expression was demonstrated in the rabbits lungs after ALI induced by LPS besides the level of cAMP and serious pathology damage of lung tissue. The result of decreased AQP5 expression was in line with the findings from Hasan and colleagues (25) that the expression of AQP5 was significantly decreased at 12 h and 24 h after treated with LPS in the mice model though the AQP5 expression was higher at 24 h than that at 12 h. However, in contrast to our results, Ohinata et al. (20) reported that the exposure of mouse lung epithelial cell line (MLE-12) to LPS at 0.5-2 h led to increased expression of AQP5 in the plasma membrane and increased osmotic water permeability. Whether the discrepancy is species-related and time-related deserves further studies. In addition, in the process of the development of ALI induced by LPS, we found that the down-regulation of AQP5 protein expression may be closely related to a large number of inflammatory mediators releasing after administration of LPS and those cytokines activating the NF-κB and/or MAPK signal pathway (8,26,27).
Once the excess water gathered in the lung tissue, the alveolar fluid needs to be reabsorbed for the epithelium to heal. Investigations had shown that β-agonists could promote the edema clearance in normal and injured lungs (28,29). In the present study, dobutamine was shown to decrease the W/D weight ratio and relieve the pulmonary edema. Additionally, the AQP5 protein expression and the cAMP level were elevated in a dose-dependent manner and the pathological damage of lung tissue was obviously improved. The above results were the evidence that dobutamine could alleviate pulmonary edema, increase AQP5 protein expression and cAMP level, which the protective effects of dobutamine on ALI were in accordance with the recent studies. Wu et al. (18) have previously reported that dobutamine may enhance alveolar fluid reabsorption by increasing the expression of AQP5 in a rat model of LPS induced lung injury. Susa and colleagues had also demonstrated that isoproterenol, a β-AR stimulant, could increase significantly AQP5 expression in rat salivary glands (30).
In the present study, we also noticed the opposite trend of AQP5 and W/D ratio both in rabbit ALI model and after the intervention of dobutamine, which we may speculate that decreased AQP5 protein expression may lead to decreased water efflux from the lung and form the pulmonary edema and eventually aggravate lung injury. Surprisingly, the above speculation was consistent with those of Zhang et al. (31), reporting that AQP5 deficiency blocked the excess water transport and damaged the pulmonary barrier function and, thus, aggravated the ALI induced by pseudomonas aeruginosa in mice model. Moreover, it is noteworthy that PaO2 and SpO2 level decreased in ALI model induced by LPS while that were improved significantly after administration of dobutamine. The variation tendency of PaO2 and SpO2 level was consistent with the pathological process of ALI. The alveolar epithelial was damaged and the permeability of alveolar-capillary barrier increased after the occurrence of ALI induced by LPS, resulting in large amounts of fluid exuding into alveolus, which affected the gas exchange of lung and finally decreased the level of PaO2 and SpO2. When the pulmonary edema was mitigated and pulmonary ventilation function was improved significantly with the administration of dobutamine, we found that the PaO2 and SpO2 level was improved obviously.
cAMP, as an intracellular second messenger, has been reported to modulate the expression of AQP5 in series of researches. Yang et al. (32) showed that the elevation of endogenous cAMP could evoke the increase in AQP5 expression level in murine lung epithelial cell line and mouse lung tissue slices. Sidhaye et al. (33) demonstrated that AQP5 expression in lung epithelial cells increased after sustained exposure to cAMP (8 h). In addition, it has been reported that cAMP-PKA signal pathway played an important role in regulating the AQP5 expression in rat cultured nasal epithelial cells (34). Indeed, PKA may rapidly induce AQP5 phosphorylation at Ser156 in BEAS-2B human bronchial epithelial cells (35) and Thr259 in human salivary gland cells and mouse salivary glands rapidly through intracellular cAMP signaling pathways (36). Similarly, β-AR agonist may increase AQP5 expression via cAMP (10,32,33). The question of why dobutamine increases the AQP5 level and decreases the pulmonary edema is undoubtedly an important consideration. Taken together with the above previous findings, our present data suggest that the regulation of AQP5 protein expression in lung tissue of rabbit ALI model may be induced via cAMP pathway.
In order to further confirm the role of cAMP pathway in regulating AQP5 protein expression in lung tissue, we examine the ICI 118,551, a specific β-2 AR antagonist, blocking the effect of dobutamine on expression of AQP5 protein and cAMP level. Surprisingly, we found that the expression of AQP5 protein and the level of cAMP in lung tissue both decreased with ICI 118,551 blocking the effect of dobutamine along with increased pulmonary edema. Therefore, we may speculate that the protective effect of dobutamine on ALI by attenuating lung edema and improving the pathological process may be dependent on the up-regulation of AQP5 via increasing intracellular cAMP level.
However, the present study has some limitations. First, we only investigated the acute effects of dobutamine on ALI within 3 h the chronic effects remain to be elucidated. Second, intravascular blood in lung may affect the accuracy of lung W/D ratio which is widely used to assess pulmonary edema. Nevertheless, in the experiment, we exsanguinated the animals carefully in all groups and did not note significant amounts of blood in the pulmonary vessels, suggested that the results could truly reflect pulmonary edema.
Conclusions
In summary, the present study has demonstrated that treatment with dobutamine significantly increase AQP5 protein expression and intracellular cAMP concentration, attenuate lung tissue W/D ratio, improve arterial blood gas analysis and the pathological process of ALI induced by endotoxin shock in rabbits in a dose-dependent manner. And ICI 118,551 could block the protective effect of dobutamine on ALI. Those results suggest that dobutamine is beneficial in the treatment of ALI and the mechanism of the protective effects of dobutamine in lung injury model may be dependent on up-regulation of AQP5 protein expression via increasing intracellular cAMP concentration. However, the exact mechanism of dobutamine on ALI needs to be further investigated before clinical application is expected.
Acknowledgements
We are grateful for the assistance of Yue Huang and Hong-gang Yi in facilitating this project.
Funding: This work was supported by grants from the Nanjing Medical Science Development Project (grant number YKK12074).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Qian F, Deng J, Gantner BN, et al. Map kinase phosphatase 5 protects against sepsis-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 2012;302:L866-74. [PubMed]
- Rump K, Brendt P, Frey UH, et al. Aquaporin 1 and 5 expression evoked by the β2 adrenoreceptor agonist terbutaline and lipopolysaccharide in mice and in the human monocytic cell line THP-1 is differentially regulated. Shock 2013;40:430-6. [PubMed]
- Hasegawa T, Azlina A, Javkhlan P, et al. Novel phosphorylation of aquaporin-5 at its threonine 259 through cAMP signaling in salivary gland cells. Am J Physiol Cell Physiol 2011;301:C667-78. [PubMed]
- Parvin MN, Kurabuchi S, Murdiastuti K, et al. Subcellular redistribution of AQP5 by vasoactive intestinal polypeptide in the Brunner's gland of the rat duodenum. Am J Physiol Gastrointest Liver Physiol 2005;288:G1283-91. [PubMed]
- Krane CM, Towne JE, Menon AG. Cloning and characterization of murine Aqp5: evidence for a conserved aquaporin gene cluster. Mamm Genome 1999;10:498-505. [PubMed]
- Shankardas J, Patil RV, Vishwanatha JK. Effect of down-regulation of aquaporins in human corneal endothelial and epithelial cell lines. Mol Vis 2010;16:1538-48. [PubMed]
- Bassnett S, Wilmarth PA, David LL. The membrane proteome of the mouse lens fiber cell. Mol Vis 2009;15:2448-63. [PubMed]
- Shen Y, Chen Z, Wang Y, et al. Aquaporin 5 expression inhibited by LPS via p38/JNK signaling pathways in SPC-A1 cells. Respir Physiol Neurobiol 2010;171:212-7. [PubMed]
- Jin Y, Yu G, Peng P, et al. Down-regulated expression of AQP5 on lung in rat DIC model induced by LPS and its effect on the development of pulmonary edema. Pulm Pharmacol Ther 2013;26:661-5. [PubMed]
- Nagai K, Watanabe M, Seto M, et al. Nitric oxide decreases cell surface expression of aquaporin-5 and membrane water permeability in lung epithelial cells. Biochem Biophys Res Commun 2007;354:579-84. [PubMed]
- Tsubota K, Hirai S, King LS, et al. Defective cellular trafficking of lacrimal gland aquaporin-5 in Sjögren's syndrome. Lancet 2001;357:688-9. [PubMed]
- Ma T, Fukuda N, Song Y, et al. Lung fluid transport in aquaporin-5 knockout mice. J Clin Invest 2000;105:93-100. [PubMed]
- Song Y, Verkman AS. Aquaporin-5 dependent fluid secretion in airway submucosal glands. J Biol Chem 2001;276:41288-92. [PubMed]
- Kabir SM, Mukherjee S, Rajaratnam V, et al. Desensitization of beta-adrenergic receptors in lung injury induced by 2-chloroethyl ethyl sulfide, a mustard analog. J Biochem Mol Toxicol 2009;23:59-70. [PubMed]
- Dhingra VK, Uusaro A, Holmes CL, et al. Attenuation of lung inflammation by adrenergic agonists in murine acute lung injury. Anesthesiology 2001;95:947-53. [PubMed]
- Hoyle GW. Mitigation of chlorine lung injury by increasing cyclic AMP levels. Proc Am Thorac Soc 2010;7:284-9. [PubMed]
- Qiu HB, Sun HM, Yang Y, et al. Effect of beta-adrenergic agonist on alveolar fluid clearance in acute lung injury: an experiment with rats. Zhonghua Yi Xue Za Zhi 2006;86:187-91. (in Chinese). [PubMed]
- Wu XM, Wang HY, Li GF, et al. Dobutamine enhances alveolar fluid clearance in a rat model of acute lung injury. Lung 2009;187:225-31. [PubMed]
- Murakami K, McGuire R, Cox RA, et al. Heparin nebulization attenuates acute lung injury in sepsis following smoke inhalation in sheep. Shock 2002;18:236-41. [PubMed]
- Ohinata A, Nagai K, Nomura J, et al. Lipopolysaccharide changes the subcellular distribution of aquaporin 5 and increases plasma membrane water permeability in mouse lung epithelial cells. Biochem Biophys Res Commun 2005;326:521-6. [PubMed]
- Szarka RJ, Wang N, Gordon L, et al. A murine model of pulmonary damage induced by lipopolysaccharide via intranasal instillation. J Immunol Methods 1997;202:49-57. [PubMed]
- Jansson AH, Eriksson C, Wang X. Lung inflammatory responses and hyperinflation induced by an intratracheal exposure to lipopolysaccharide in rats. Lung 2004;182:163-71. [PubMed]
- Lin Y, Zhu X, Yao WZ, et al. Yohimbine protects against endotoxin-induced acute lung injury by blockade of alpha 2A adrenergic receptor in rats. Chin Med J (Engl) 2011;124:1069-74. [PubMed]
- Bosmann M, Grailer JJ, Zhu K, et al. Anti-inflammatory effects of β2 adrenergic receptor agonists in experimental acute lung injury. FASEB J 2012;26:2137-44. [PubMed]
- Hasan B, Li FS, Siyit A, et al. Expression of aquaporins in the lungs of mice with acute injury caused by LPS treatment. Respir Physiol Neurobiol 2014;200:40-5. [PubMed]
- Yao C, Purwanti N, Karabasil MR, et al. Potential down-regulation of salivary gland AQP5 by LPS via cross-coupling of NF-kappaB and p-c-Jun/c-Fos. Am J Pathol 2010;177:724-34. [PubMed]
- Shen Y, Wang X, Wang Y, et al. Lipopolysaccharide decreases aquaporin 5, but not aquaporin 3 or aquaporin 4, expression in human primary bronchial epithelial cells. Respirology 2012;17:1144-9. [PubMed]
- Saldías FJ, Lecuona E, Comellas AP, et al. beta-adrenergic stimulation restores rat lung ability to clear edema in ventilator-associated lung injury. Am J Respir Crit Care Med 2000;162:282-7. [PubMed]
- Mutlu GM, Dumasius V, Burhop J, et al. Upregulation of alveolar epithelial active Na+ transport is dependent on beta2-adrenergic receptor signaling. Circ Res 2004;94:1091-100. [PubMed]
- Susa T, Sawai N, Aoki T, et al. Effects of repeated administration of pilocarpine and isoproterenol on aquaporin-5 expression in rat salivary glands. Acta Histochem Cytochem 2013;46:187-97. [PubMed]
- Zhang ZQ, Song YL, Chen ZH, et al. Deletion of aquaporin 5 aggravates acute lung injury induced by Pseudomonas aeruginosa. J Trauma 2011;71:1305-11. [PubMed]
- Yang F, Kawedia JD, Menon AG. Cyclic AMP regulates aquaporin 5 expression at both transcriptional and post-transcriptional levels through a protein kinase A pathway. J Biol Chem 2003;278:32173-80. [PubMed]
- Sidhaye V, Hoffert JD, King LS. cAMP has distinct acute and chronic effects on aquaporin-5 in lung epithelial cells. J Biol Chem 2005;280:3590-6. [PubMed]
- Wang W, Zheng M. Role of cAMP-PKA/CREB pathway in regulation of AQP 5 production in rat nasal epithelium. Rhinology 2011;49:464-9. [PubMed]
- Woo J, Chae YK, Jang SJ, et al. Membrane trafficking of AQP5 and cAMP dependent phosphorylation in bronchial epithelium. Biochem Biophys Res Commun 2008;366:321-7. [PubMed]
- Kumari SS, Varadaraj M, Yerramilli VS, et al. Spatial expression of aquaporin 5 in mammalian cornea and lens, and regulation of its localization by phosphokinase A. Mol Vis 2012;18:957-67. [PubMed]


