|
Original Article
Palliative Hypofractionated Radiotherapy For Non-small-cell Lung Cancer(NSCLC) Patients Previously Treated By Induction Chemotherapy
George A. Plataniotis, Maria-Aikaterini Theofanopoulou, Konstantinia Sotiriadou, Kyriaki Theodorou, Panagiotis Mavroidis, George Kyrgias
From Departments of Oncology (Dr Plataniotis, current affiliation), A berdeen Royal Infirmary, UK;
Departments of Radiation Oncology (Drs Sotiriadou and Kyrgias), University Hospital of Larissa, GREECE;
Departments of Medical Physics (Drs Theodorou and Mavroidis), University Hospital of Larissa, GREECE
Corresponding to: George A. Plataniotis, MD, PhD, Department of Oncology, Aberdeen Royal Infirmary, Foresterhill AB25 2ZN, Aberdeen, UK. Tel: +44-1224 553483, Fax: +44-1224-554183. E-mail: george.plataniotis@nhs.net
|
|
Abstract
Aim:
To investigate the effectiveness and toxicity of radiotherapy (RT) given as 17 Gy in 2 fractions, in patients with locally advanced
non-small-cell lung cancer (NSCLC) previously treated by platinum-based chemotherapy (CHT) and the impact of total tumor volume
(TTV) on symptoms control
Materials and methods:
Patients with inoperable NSCLC resistant to induction platinum-based CHT, who developed symptoms during
or just after radiotherapy, were treated by 17 Gy in two fractions one week apart. In 12/28 patients a minimal response (up to 20% of TTV)
and in 16/28 a stable or locally progressive disease had been recorded after induction CHT. In 26/28 patients, symptoms were present dur-
ing-after CHT and before RT. The prognostic significance of pre-RT TTV on symptoms control and patients survival was also examined.
Results:
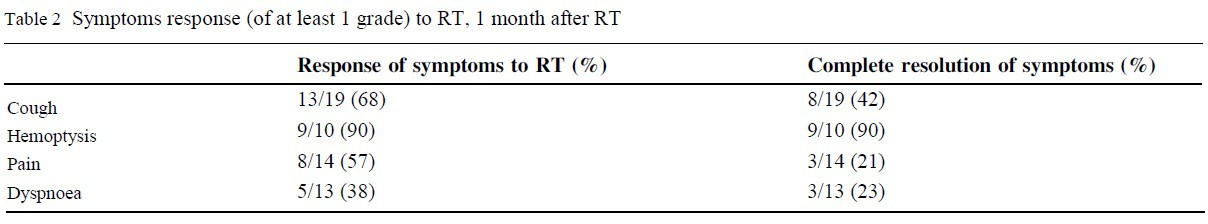
We report on 28 patients. Response rates for the four main symptoms were: cough 13/19 (68%), haemoptysis 9/10 (90%), pain
8/14 (57%) and dyspnoea 5/13 (38%). Hematologic and local-thoracic toxicities were minimal. The 0median survival from the beginning of
RT, for the whole group of patients was 9 months (95% CI:3.7-14.3), while for those patients with TTV<120 cc it was 12 months, and for
those with TTV 120cc, it was 5.2 months. TTV was not suggested to influence symptoms control rate.
Conclusion:
The two-fraction radiotherapy course is safe and effective in palliation of symptomatic non-small-cell lung cancer patients
non-responding to induction CHT. Present data suggests that the TTV may influence survival time.
Key words
Non-small-cell lung cancer; chemotherapy; radiotherapy; palliation; fractionation J Thorac Dis 2009;1:5-10. DOI: 10.3978/j.issn.2072-1439.2009.12.01.005
|
|
Introduction
Patients with non-small cell lung cancer (NSCLC) can be divid-
ed into three groups ( 1). Those with good performance status and a
small/localised tumour, who are candidates for radical treatment;
those with poor PS and advanced or metastatic tumour for whom
simple palliative measures only are appropriate; and a middle
group of patients (who are the majority) with (Karnofsky Performance Status) KPS equal or higher than 70 and locally advanced
(stage III) disease. Within this last group, the borders between
treatment with radical and palliative intent are ill defined and the
treatment of these people remains controversial. Radiotherapy
(RT) and chemotherapy (CHT) have been used in various combinations (concurrently, sequentially etc) for this group of patients( 2). In some randomised trials such as those from Cancer and
Leukemia Group B (CALGB 8433) ( 3) and the Radiation Therapy
Oncology Group (RTOG 8808) ( 4) induction (neo-adjuvant)
chemotherapy has been used for these patients. Patients responding
to initial CHT are candidates for further treatment by radical RT or
concurrent RT-CHT ( 5). Nevertheless, as the response rates after
induction CHT are approximately 30-40% ( 6) there is a significant
amount of patients not responding or relapsing/progressing or even
suffering worsening of their symptoms during or after CHT. Although second-line CHT, is gaining acceptance ( 7, 8), some of
these patients develop serious symptomatology and worsening of
their performance status and are referred for RT.
The RT schedule of 17 Gy in 2 fractions, one week apart is ef-
fective for symptoms palliation in previously untreated patients
with locally advanced NSCLC ( 9- 21). In addition, it is convenient
for patients (especially those coming from remote areas with a difficult access to RT facility) and accelerates patients turnover in RT
department.
We have reported our experience on palliative hypofractionated
RT for previously untreated patients with locally advanced NSCLC
( 19). The present (not prospectively designed) study is a report on
our recorded experience (from Radiotherapy Department in Uni
versity Hospital of Larissa, Greece) with this RT schedule on simi
lar patients not responding to induction CHT, who develop uncon
trollable symptoms during or just after it. To our knowledge there
is no study reporting feasibility and toxicity of this RT schedule after induction CHT.
As the tumor volume has been reported to influence prognosis
in lung ( 21, 22) head and neck ( 24) and other carcinomas ( 25), we
have attempted to investigate the prognostic impact of pre-RT tumor volume on patients' symptoms control and survival.
|
|
Material and methods
Patient population
Patients with locally advanced, inoperable, stage IIIA-B
NSCLC, were treated by platinum-based induction chemotherapy
with the intend to be treated by concurrent RT-CHT or radical
RT-alone, thereafter. Patients were evaluated after 3 cycles of
CHT. Evaluation was based on clinical picture and chest CT scan.
Those with a response to the initial 3 cycles of chemotherapy, were
planned to receive 3 more cycles aiming at maximal tumor response. Patients who developed at least one of the following during or just after the completion of CHT, were referred for RT: lo-
cally progressive or non-responding disease (see below on how response was assessed), progressive symptomatology, lowering of
KPS, weight loss of >10% in the past 3 months, presence of symptomatic pleural effusion. Symptoms included at least one of the
following: chest pain, dyspnoea-wheezing, cough and haemoptysis.
Patients with superior vena cava obstruction were not included in
this group of patients as they were treated by more protracted RT
schedules, such as 20 Gy in 4 fractions or 30 Gy in 10 fractions.
All patients had histologically or cytologically confirmed NSCLC
and complete initial (before CHT) staging with clinical examination, bronchoscopy, thoracic, upper abdominal and brain CTs,
bone scan, and laboratory tests. Before starting RT, a chest CT
scan was requested; restaging was carried out in case of clinical
suspicion for metastatic disease.
Treatment
Radiotherapy was given in a median time of 5 weeks after the
last cycle of chemotherapy. All patients underwent conventional
simulation before treatment. The RT portals were delineated to encompass the GTV (Gross Tumour Volume) with 1-2 cm margin to
all directions. An AP/PA parallel pair of fields was employed in the
most of the patients (25/28). In 3/28 of the patients, opposed
oblique fields were used. Field sizes varied between 8x7 to 13x13
cm 2 (median field surface 120 cm 2). A spinal cord block 2.5 cm in
width was added to the posterior field of the second fraction. With
this technique we had not observed any radiation myelitis in the
past ( 26). RT consisted of 17 Gy in two fractions one week apart.
An isocentric technique (with lung correction) was used in all patients and the dose was prescribed at the isocenter. Filter compensators were used if needed to maintain the calculated dose within
7% of the prescribed dose. Linear accelerator delivering 6 MV
photons was used.
Evaluation of the patients
Palliation of symptoms within 3 months after the end of RT, and
toxicity were recorded. The patients were followed-up 1 and 3
months after RT and every 3 months thereafter. Follow-up examinations included detailed history, clinical examination, blood tests,
as well as a check for metastatic disease, if clinically indicated.
Chest radiograph was obtained at 3 months after RT. Questionnaires for the assessment of quality of life were not used due to
poor patients' compliance in fulfilling the forms ( 19).
The symptoms assessed included cough, dyspnoea, chest pain
and haemoptysis; a four-degree categorical scale was used for each
of the main symptoms: none 0, mild 1, moderate 2, and severe 3.
Especially for dyspnoea the scale was as follows: 0: walks without
dyspnoea, 1: walks with mild dyspnoea, 2: dyspnoea on walking a
short distance and 3: dyspnoea with mild exertion ( 19).
Symptoms palliation and treatment-related toxicity were assessed and recorded by the radiation oncologists who scored symptoms relief according to patient's statement. Symptoms were graded
and recorded at the first day of RT and at every patient's visit during follow-up time. Symptomatic response was assessed by comparing the initial score for each symptom with the best score during
the first 3 months of follow-up. An improvement of one grade or
higher was considered as response. A total symptom score (TSS)
was produced for each patient, by adding the scores of each individual symptom. All patients were able to visit hospital at one and
3 months for follow-up evaluation.
Toxicities assessed and recorded at each follow-up visit, includ
ed: anorexia/nausea-vomiting, skin reaction, pneumonitis, esophagitis, hematological toxicity and radiation myelopathy. Because of
the preceding cytotoxic chemotherapy, all patients were surveyed
closely and were advised either to visit us or to telephone in case of
serious toxicity in the meantime between their prebooked attendances. For the grading of toxicity the RTOG acute/late radiation
morbidity scoring was used.
CT evaluation-volumetry
The pre-CHT and pre-RT CT scans were reviewed. Examinations were obtained with various CT machines. All imaging studies were performed with IV injection of contrast medium. A slice thickness of 5-10 mm was used in all cases. Soft tissue windows were used for defining mediastinal masses and lung windows were used for lesions surrounded by lung parenchyma.
To determine tumour volume, the primary tumour and enlarged (>1.5 cm) lymph nodes were outlined on each CT slice that
contained tumour. Tumour outlines were then transferred into a
treatment-planning computer using a digitiser. After accounting for
the magnification factor and CT slice thickness, the computer generated a tumour volume measured in cubic centimetres. The
total tumour volume was registered for each patient: primary
tumour plus enlarged lymph nodes.
Patients were evaluated after CHT according to RECIST
(Response Evaluation Criteria In Solid Tumors) criteria ( 27).
According to RECIST criteria partial response is defined as
a decrease of at least 65% in tumor volume. The response
after CHT was evaluated by the ratio (R) of volumes:
Stable disease was characterized in case of R=1 (0.05);
"minimal response" if R >1.05, and progressive disease if
R < 0.95 (please note that the TTV as measured just after
chemotherapy is actually assigned as pre-RT TTV).
Statistical analysis
Descriptive statistics and simple proportions were used to
present the data. The two patients with stage IIB were
grouped in IIIA stage for the purpose of analysis. Statistical
analysis was undertaken using the Wilcoxon signed-rank test
and chi-squared non-parametric tests. Correlations were
studied by Pearson correlation coefficient. Overall survival
was estimated, from the date of starting RT, by the Kaplan-Meier method. Patients alive at the last follow-up were
censored. The prognostic cut-off point in TTV was searched
for the value that would yield the best discrimination between the two Kaplan-Meier survival curves, as assessed by
the repeated application of the Log-rank test. We used the
correction as proposed by Altman et al ( 28), and give both
values. Univariate survival analysis was performed using the
log-rank test. The parameters examined (given the small
number of patients in the study) were: KPS as a binary variable (>70 vs. 70), total tumor volume (TTV) as both continuous and binary (<120 vs. >120 cc) variable and response
to chemotherapy as a binary variable (minimal response vs.
no response or progressive disease). The impact of factors
on patients survival was studied with the Cox proportional
hazards model (backward stepwise selection routine) ( 29).
|
|
Results
Patients and treatment
Although our study is not prospectively designed, it includes patients treated according to a particular radiotherapy
clinical protocol. Between March 2003 and November 2004,
28 patients were treated. Their characteristics are shown in
table 1. Four patients had received 4 cycles of docetaxel as a
second-line CHT, before RT. There were also patients with
symptoms worsening after 1-2 cycles and they were also referred for RT. Two patients declined further chemotherapy; they
both had stable disease after 3 cycles of CHT.
In detail, induction chemotherapy consisted of one of the following combinations: 3 cycles of paclitaxel+carboplatin (n=4), 3
cycles of paclitaxel+carboplatin followed by docetaxel (n=4), 1 cycle of gemsitabine+carboplatin (n=2), 3 cycles of gemsitabine+carboplatin (n=4), 6 cycles of gemsitabine+carboplatin (n=4), 3 cycles
of gemsitabine+cisplatin (n=2), 6 cycles of gemsitabine+cisplatin
(n=4), 2 cycles of paclitaxel-cisplatin (n=2). One patient received 3
cycles of cisplatin+vinorelbine and one 3 cycles of taxotere+vinorelbine.
Symptoms control and survival
According to RECIST criteria ( 27) all of our patients were
non-responders to chemotherapy. Eight patients had stable disease,
8 had progressive disease and, 12 had "minimal response"
(~15-20% decrease in tumor volume). The response of each symptom to RT is presented in table 2.
Hemoptysis was the symptom with the most remarkable response rate after RT (9/10 patients, 90%). The TSS before RT was
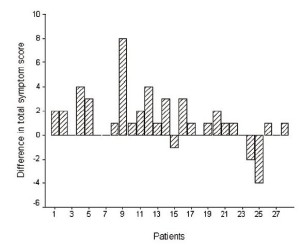
significantly higher to that after RT (Wilcoxon signed-rank test
Z=-2.894, p=0.004) ( fig.1). After RT the TSS was lower (improvement) in 19 patients (68%), stable in 6 (21%), and higher in 3
(11% ) patients. KPS was stable in 17 patients, lower in 7 and
higher in 4 patients (Wilcoxon signed-rank test Z=-1.182, p=0.237). The rates of complete symptoms resolution were: cough 8/19
(42%), hemoptysis 9/10 (90%), dyspnoea 3/13 (23%), chest pain
3/14 (21%).
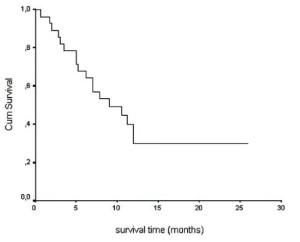
From the beginning of RT the median survival time for the
whole group of patients was 9 months (95% CI: 3,7-14.3) and
one-year survival rate was 29.8% (SE: 0,956) ( fig. 2).
Volumes and correlations
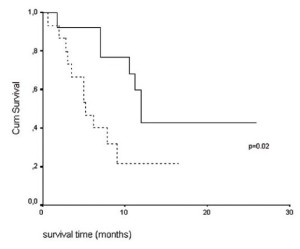
The median and mean tumor volumes were 129.9 and 131.5
cm 3 respectively. Patients with tumor volume 120 cm 3 had a median survival of 5.2 months (95% CI: 2.6-7.7), while those with tumor volume <120 cm 3 had a median survival of 12 months (95%
CI: 10.7-13.3) (log-rank test, p=0.02 and after correction ( 28)
pcorr=0.09) ( fig. 3). Tumor volume has shown no association with
the parameter "difference in TSS" (before and after RT), (Pearson
correlation coefficient, p=0.7) i.e. the degree of palliation could not
be related with tumor volume, in the present material.
Prognostic factors for survival
Parameters significant for survival in univariate analysis were:
tumor volume as a binary variable more or less than 120 cm3 (see
above), response to chemotherapy (minimal vs. all others) (p=0.03)
and KPS as a binary variable (p=0.048). In Cox regression analysis
important variables were: tumor volume as a dichotomous variable
(Hazard Ratio, HR:0.23, 95% CI:0.075-0.7, p=0.007), and re
sponse to chemotherapy (HR:0.24, 95%CI: 0.08-0.75, p=0.008).
Toxicity
Esophagitis toxicity Grade 3-4 was not seen; seven patients had
a Grade 2 and 8 patients a Grade 1 esophagitis. Radiation pneumonitis occurred in 2 patients and subsided promptly (within 7-10
days) after dexamethazone administration. Dyspnoea was recorded
as "worse" after RT for these patients. Fatigue was reported in
11/28 (39%) and nausea/vomiting in 6/28 (21%) during the first
week after RT. No radiation myelitis was recorded up to the end of
the follow-up time. The four patients treated with second-line CHT
before RT did not develop excessive side effects.
|
|
Discussion
After the publication of the MRC (Medical Research Council)
studies in the UK ( 9, 10) on hypofractionated RT in locally advanced NSCLC, many centers around the world have adopted it
( 11- 15, 17- 21), while others have criticized hypofractionation with
1-2 fractions ( 30). The criticism for this hypofractionation is
two-fold: firstly it is focused on the issue that higher-dose regimenwould probably offer an increase in survival or a more durable response and, secondly there is concern about the potential toxicity
of large dose per fraction. In the USA although hypofractionated
RT schemes have been used from time to time, radiation oncologists are generally reluctant to prescribe such a hypofractionated
treatment for lung cancer. A recent study of a 2 x 8.5 Gy RT
course, from Boston ( 12) has been terminated after an accrual of
only 23 patients in a time interval of 7 years, because the doctors
"did not want to deny fit patients potentially curative treatment or
treatment that might give a more durable response". They only
treated patients, with ECOG Performance Sstatus of 2 or worse or those that could not tolerate a more aggressive treatment course.
However, their clinical results were comparable to those reported
by other similar studies.
A randomised MRC trial ( 31) offers a strong evidence of a
modest increase in survival (5% at 1 year and 3% at 2 years) in patients with better PS, who were treated by 12-13 fractions of 3 Gy.
Other studies have also favored more protracted RT schedules ( 14, 16, 18). However in the most recent randomised study (with 421
patients) from Norway ( 13) it was shown that protracted palliative
RT of 42 Gy in 15 fractions or 20 Gy in 25 fractions were not superior to the 17 Gy in 2 fractions regimen, in terms of symptoms control and survival.
Toy et. al. in an interesting review of the literature, have concluded that symptomatic patients with NSCLC can be treated safely and effectively with regimens of RT of one or two fractions.
Nevertheless selected patients with good PS could be considered
for higher-dose regimens if the chance of modest improvement in
survival and palliation is considered worth the additional inconvenience and toxicity ( 21).
In the present study the median survival from the beginning of
RT was higher (9 months) from that reported by hypofractionated
RT-alone studies, probably due to both patients selection before
CHT and a likely additive effect of CHT and RT ( 1).
The finding that the patients with total tumour volume (after
CHT) of less than 120 cc had a median survival of 12 months,
while those with TTV>120 cc had a median overall survival of only 5.2 months (log-rank, p=0.02), might be of importance.
In a report by Willner et al. ( 22) from the University of
Wü rzburg the authors concluded that tumors 100 cm3 were unlikely to be
controlled long term, but tumor volume was not significant for survival in their study. A similar cut-off value for tumor
control has been reported in a series of 22 patients with NSCLC
( 23). Impact of TTV and its reduction during or after RT has been
examined by some recent studies ( 32- 34).
The RT schedule of 17 Gy in 2 fractions seems to be safe and it
offers a reasonable palliation rate of symptoms for patients previously treated by platinum-based CHT. Although initial tumor volume was not suggested to affect the rate of symptoms palliation,
could be a criterion for patients stratification in future studies.
|
|
References
-
Hoskin PJ. Palliative Radiotherapy for Non-small-cell Lung Cancer: Which Dose?(Editorial). Clin Oncol 2005;17:59–60.
[LinkOut]
-
Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell
lung cancer: a meta-analysis using updated data on individual patients from 52
randomised trials. BMJ 1995;311:899–909.
[LinkOut]
-
Dillman RO, Herndon J, Seagren SL, Eaton WL Jr, Green MR. Improved survival
in stage III non-small-cell lung cancer: seven-year follow-up of cancer and
leukemia group B (CALGB) 8433 trial. J Natl Cancer Inst 1996;88:1210–5.
[LinkOut]
-
Sause WT, Scott C, Taylor S, Johnson D, Livingston R, Komaki R, et al. Radiation
therapy oncology group (RTOG) 88–08 and eastern cooperative oncology group
(ECOG) 4588: preliminary results of a phase III trial in regionally advanced, unre-
sectable non-small-cell lung cancer. J Natl Cancer Inst 1995;87:198–205.
[LinkOut]
-
Saunders MI, Rojas A, Lyn BE, Wilson E, Phillips H. Dose-escalation with
CHARTWEL (Continuous Hyperfractionated Accelerated Radiotherapy Week-End
Less) Combined with Neo-adjuvant Chemotherapy in the Treatment of Locally
Advanced Non-small Cell Lung Cancer. Clinical Oncology 2002;14:352–60.
[LinkOut]
-
Shepherd FA. Chemotherapy for non-small cell lung cancer: Have we reached a
new plateau? Semin Oncol 1999;26:3-11.
[LinkOut]
-
Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O'Rourke M, et al.
Prospective randomised trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based
chemotherapy. J Clin Oncol 2000;18:2095-103.
[LinkOut]
-
Buccheri G, Ferrigno D. Second-line weekly paclitaxel in patients with inoperable
non-small cell lung cancer who fail combination chemotherapy with cisplatin.
Lung Cancer 2004;45:227-36.
[LinkOut]
-
Anonymous. Inoperable non-small-cell lung cancer (NSCLC): a medical Research
Council randomized trial of palliative radiotherapy with two fractions or ten frac-
tions. Report to the Medical Research Council by its Lung Cancer Working Party.
Br J Cancer 1991;63:265–70.
[LinkOut]
-
Anonymous. A Medical Research Council (MRC) randomized trial of palliative
radiotherapy with two fractions or a single fraction in patients with inoperable
non-small-cell lung cancer (NSCLC) and poor performance status. Medical Re-
search Council Lung Cancer Working Party. Br J Cancer 1992;65:934–41.
[LinkOut]
-
Rees GJ, Devrell CE, Barley VL, Newman HF. Palliative radiotherapy for lung
cancer: two versus five fractions. Clin Oncol 1997;9:90–5.
[LinkOut]
-
Cross CK, Berman S, Buswell L, Johnson B, Baldini EH et al. Prospective study
of palliative hypofractionated radiotherapy (8.5 Gy x 2) for patients with symp-
tomatic non–small-cell lung cancer. Int Jour Radiat Oncol Biol Phys 2004;58:1098–105.
[LinkOut]
-
Sundstrom S, Bremnes R, Aasebo U, Aamdal S, Hatlevoll R, Brunsvig P et al.
Hypofractionated palliative radiotherapy (17 Gy per two fractions) in advanced
non-small-cell lung carcinoma is comparable to standard fractionation for symp-
tom control and survival: a national phase III trial. J Clin Oncol 2004;22:801–
10.
[LinkOut]
-
Bezjak A, Dixon P, Brundage M, Tu D, Palmer MJ, Blood P et al. Randomized
phase III trial of single versus fractionated thoracic radiation in the palliation of
patients with lung cancer (NCIC CTG SC.15). Int J Radiat Oncol Biol Phys 2002;54:719–28.
[LinkOut]
-
Kramer G, Wander S, Noordijk E. Randomized Dutch National Study of the ef-
fects of irradiation with different treatment schedules in the palliation of
non-small-cell lung cancer (NSCLC). Lung Cancer 2003;41:S38.
[LinkOut]
-
Erridge SC, Gaze MN, Price A, Kelly CG, Kerr GR, Cull A, et al. Symptom Control and Quality of Life in People with Lung Cancer: A Randomized Trial of Two
Palliative Radiotherapy Fractionation Schedules. Clin Oncol 2005;17:61–7.
[LinkOut]
-
Nestle U, Nieder C, Walter J, Abel U, Ukena D, Sybrecht GW, Schnabel K. A
palliative accelerated irradiation regimen for advanced non-small-cell lung cancer
vs. conventionally fractionated 60 Gy: Results of a randomized equivalence
study. Int J Radiat Oncol Biol Phys 2000;48:95–103.
[LinkOut]
-
Gaze MN, Kelly CG, Kerr GR, Cull A, Cowie VJ, Gregor A, et al. Fractionated
thoracic radiotherapy gives better symptom relief in patients with non-small cell
lung cancer. EJC 2001;37:S29(suppl 6).
-
GA Plataniotis, JR Kouvaris, C Dardoufas, Kouloulias V, Theofanopoulou MA,
Vlahos L. A short radiotherapy course for locally advanced non-small cell lung
cancer (NSCLC). Effective palliation and patients’convenience. Lung Cancer
2002;35:203-7.
[LinkOut]
-
Stevens MJ, Begbie SD. Hypofractionated irradiation for inoperable non-small
cell lung cancer. Australas Radiol 1995;39:265-70.
[LinkOut]
-
Toy E, Macbeth F, Coles B, Melville A, Eastwood A. Palliative thoracic radioptherapy for non-small-cell lung cancer. Am J Clin Oncol 2003;26:112-20.
[LinkOut]
-
Willner J, Baier K, Caragiani E, Tschammler A, Flentje M. Dose, volume and tumor control predictions in primary radiotherapy of non-small-cell lung cancer. Int
Jour Radiat Oncol Biol Phys 2002; 52: 382-9.
[LinkOut]
-
Werner-Wasik M, Xiao Y, Pequignot E, Hauck W. Assessment of lung cancer response after nonoperative therapy: tumor diameter, bidimensional product, and
volume. A serial CT scan-based study. Int Jour Radiat Oncol Biol Phys 2001;51:56-61.[LinkOut]
-
Plataniotis GA, Theofanopoulou ME, Kalogera-Fountzila A, Haritanti A,
Ciuleanou E, Ghilezan N, et. al. The prognostic impact of tumor volumetry in patients with locally advanced head and neck carcinomas (non-nasopharyngeal)
treated either by radiotherapy alone or by combined radiochemotherapy in a randomized trial. Int Jour Radiat. Oncol Biol Phys 2004;59:1018-26.
[LinkOut]
-
Mayr NA, Magnotta VA, Ehrhardt JC, Wheeler JA, Sorosky JI, Wen BC, et al.
Usefulness of tumor volumetry by magnetic resonance imaging in assessing response to radiation therapy in carcinoma of the uterine cervix. Int J Radiat Oncol
Biol Phys 1996; 35:915-24.[LinkOut]
-
Plataniotis GA, Kouvaris JR, Dardoufas C, Pistevou-Gobaki K, Kouloulias V, Papadopoulos LS, Vlahos L. A short course of palliative radiotherapy for inoperable
NSCLC: biologically effective dose to the spinal cord. Clin Oncol 2000;12:335.
[LinkOut]
-
Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer
treatment. Cancer 1981;47:207–14.[LinkOut]
-
Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using‘‘optimal’’cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst 1994;86:829–35.
[LinkOut]
-
Cox D: Regression models and life tables (with discussion). J Royal Stat Soc B1972;34:187-220.
[LinkOut]
-
Bogart JA Hypofractionated Radiotherapy for Advanced Non–Small-Cell Lung
Cancer: Is the LINAC Half Full? J Clin Oncol 2004;22:765-8.
[LinkOut]
-
Macbeth FR, Bolger JJ, Hopwood P, Bleehen NM, Cartmell J, Girling DJ, et al.,
for the Medical Research Council Lung Cancer Working Party. Randomised trial
of palliative two-fraction versus more intensive 13-frac-tion radiotherapy for patients with inoperable non-small cell lung cancer and good performance status.
Clin Oncol 1996;8:167–75.
[LinkOut]
-
Seibert RM, Ramsey CR, Hines JW, Kupelian PA, Langen KM, Meeks SL, Scaperoth DD. A model for predicting lung cancer respon?se to therapy. Int J Radiat
Oncol Biol Phys 2007;67:601-9.
[LinkOut]
-
Siker ML, Tome WA, Mehta MP. Tumor volume changes on serial imaging with
megavoltage CT for non-small-cell lung cancer during intensity-modulated radiotherapy: How reliable, consistent, and meaningful is the effect? Int J Radiat Oncol
Biol Phys 2006;66:135–41.
[LinkOut]
-
Bosmans G, van Baardwijk A, Dekker A, Ollers M, Boersma L, Minken A, et al.
Intra-patient variability of tumor volume and tumor motion during conventionally
fractionated radiotherapy for locally advanced non small-cell lung cancer: A
prospective clinical study. Int J Radiat Oncol Biol Phys 2006;66:748–53.
[LinkOut]
Cite this article as: Plataniotis GA, Theofanopoulou MA, Sotiriadou K, Theodorou K, Mavroidis P, Kyrgias G. Palliative Hypofractionated Radiotherapy For Non-small-cell Lung Cancer(NSCLC) Patients Previously Treated By Induction Chemotherapy. J Thorac Dis 2009;1:5-10. doi: 10.3978/j.issn.2072-1439.2009.12.01.005
|