
Detrimental effects of elevated transpulmonary gradient on outcomes following restrictive mitral annuloplasty in patients with pre-existing pulmonary hypertension
Introduction
Both ischemic and nonischemic dilated cardiomyopathy are frequently complicated by functional mitral regurgitation (MR) consequent to progressing left ventricular (LV) remodeling (1). Medically refractory severe functional MR has a strong negative impact on the survival of patients with advanced heart failure (2). Restrictive mitral annuloplasty (RMA) that utilizes an undersized prosthetic ring is effective for surgical treatment of patients with end-stage cardiomyopathy and medically refractory functional MR. Previous studies have demonstrated that RMA can eliminate functional MR, promote partial LV reverse remodeling, and improve functional capacity and symptoms (3-6).
Pulmonary hypertension (PH) is a well-known indicator of poor prognosis in patients with dilated cardiomyopathy (7-10). In patients with chronic LV dysfunction, PH primarily results from a “passive” backward transmission of elevated left-sided filling pressure owing to systolic or diastolic LV dysfunction (11,12). However, in patients with long-standing heart failure and chronic MR, chronically elevated LV filling pressure can lead to the development of pulmonary vascular disease with vasoconstriction and remodeling of the pulmonary arterial bed (13). This “reactive” form of PH is manifested as an increased pulmonary vascular resistance (PVR) and transpulmonary pressure gradient (TPG) (TPG >12 mmHg) that is superimposed on pulmonary venous pressure (14,15).
It has been reported that TPG can be used as a parameter to predict outcomes in patients with advanced cardiomyopathy. Chatterjee et al. identified an independent association between high TPG (≥12 mmHg) and significantly worse clinical outcomes in patients with systolic heart failure who met standard indications for cardiac resynchronization therapy (16). Notably, there were no significant differences in clinical outcomes between patients without PH and patients with PH but normal TPG. Additionally, Erickson et al. reviewed preoperative pulmonary hemodynamic parameters and clinical outcomes of 109 patients who underwent heart transplantation and found that elevated preoperative TPG (≥12 mmHg) was associated with a higher risk of mortality after orthotopic heart transplantation (17). Interestingly, systolic PA pressure and PVR did not accurately predict a poor outcome after orthotopic heart transplantation. Likewise, Murali et al. evaluated the influence of preoperative TPG and PVR on early post-transplant mortality in 425 orthotopic transplant recipients. They found that preoperative TPG, but not preoperative PVR, was independently associated with post-transplant early mortality (18). These findings highlight the prognostic role of invasive assessment of pre- and post-treatment TPG in patients with end-stage cardiomyopathy as consequences of pulmonary vascular disease.
The negative impact of elevated TPG on clinical outcomes is mainly due to the inability of the myocardium to adapt to right ventricular pressure overload and progressive pulmonary vascular disease. Despite the growing recognition of the clinical importance of pulmonary vascular disease in LV systolic dysfunction, its contribution to clinical outcomes after an RMA procedure remains unknown. Additionally, whether the reactive form of PH (i.e., TPG >12 mmHg) affects post-RMA pulmonary hemodynamics in patients with severely impaired LV function and pre-existing PH remains unknown. Thus, this study aims to determine the reversibility of pulmonary vascular hemodynamics over time following RMA in patients with pre-existing PH and with a focus on preoperative TPG. The association of preoperative elevated TPG with late outcomes in patients with severe LV dysfunction was also determined. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-2898).
Methods
Patients
The records of 147 patients with pre-existing PH, defined as mean pulmonary artery pressure (PAP) of ≥25 mmHg at rest as assessed using right heart catheterization, and who underwent RMA for functional MR secondary to ischemic or non-ischemic advanced cardiomyopathy (ejection fraction of ≤40%) between 1999 and 2010 were examined. Of these records, patients whose postoperative hemodynamic data were not available (n=67), required redo mitral valve surgery (n=2), required an LV assist device implantation postoperatively (n=3), or who were not followed up for more than one year after RMA (n=11) were excluded. Thus, 64 patients were finally enrolled. Each had functional MR secondary to LV remodeling and systolic restrictive motion of the mitral leaflets upon echocardiography (Carpentier classification type IIIb).
All patients underwent RMA via median sternotomy under mild hypothermic cardiopulmonary bypass as well as meticulous myocardial preservation with antegrade and retrograde intermittent cold blood cardioplegia. No other adjunct procedures were performed on the mitral valve itself. The degree of downsizing was determined not only according to the annular geometry of the native valve, but also according to the body surface area of the patient. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Osaka University (IRB number 08218-6), and the need for written informed consent from the patients was waived.
Cardiac catheterization and definition
Pre- and postoperative (1 month) cardiac catheterization were performed in all patients. Hemodynamic data, namely cardiac output (assessed using thermo-dilution method), mean PAP, pulmonary capillary wedge pressure (PCWP), TPG (calculated as mean PAP minus PCWP), PVR (calculated as 80× TPG/cardiac output), and systemic vascular resistance (calculated as 80 × (mean aortic pressure minus right atrial pressure)/cardiac output) were obtained.
Echocardiography
Two-dimensional and Doppler transthoracic echocardiography examinations were performed one week prior to the surgery (baseline), 1 month after surgery, and annually thereafter. Anatomic and Doppler measurements were performed according to the recommendations of the American Society of Echocardiography. Regurgitation severity was classified as none (0), trivial (1+), mild (2+), moderate (3+), or severe (4+). Systolic PAP was calculated by adding the systolic pressure gradient across the tricuspid valve derived from tricuspid regurgitation to the estimated right atrial pressure value. The dimension of the inferior vena cava (IVC) was measured using a subcostal approach.
Outcomes and clinical follow-ups
The primary outcome was a change in pulmonary hemodynamics after RMA, while the secondary outcome was composite adverse events, defined as all-cause mortality and first readmission for heart failure. Diagnosis of postoperative recurrent heart failure was based on clinical symptoms, physical signs, or radiological evidence of pulmonary congestion.
Statistical analysis
Patients were classified into two groups based on preoperative TPG: low (≤12 mmHg, n=45) and elevated TPG (>12 mmHg, n=19) (14,15). Continuous variables were expressed as medians with interquartile ranges (IQR) and compared using a Mann-Whitney U test for unpaired data and a Wilcoxon signed rank sum test for paired data. Categorical variables were expressed as frequencies and proportions and were compared using the chi-squared test or Fisher’s exact test, respectively. Hemodynamic data were analyzed using the analysis of covariance model, including factors for the corresponding baseline value as the covariate, group, and interaction between them. Echocardiographic data (LV dimension, LV ejection fraction, left atrial (LA) dimension, systolic PAP, and IVC dimension) obtained over time after surgical intervention were analyzed using a mixed-effects model for repeated measures, including factors for the corresponding baseline value, group, time, and interaction between them. The variance-covariance matrix of the observation using a linear mixed-effects model was assumed to be unstructured. Assessment time points were treated as categorical factors. Correlations between continuous variables were tested with Spearman’s rank correlation (rho).
The linearized mortality rate was determined by dividing the number of patients experiencing an event by patient years at risk. The survival analysis was performed using the Kaplan-Meier method for estimations and the log-rank test. The associations between preoperative factors and composite adverse events were examined using the Cox proportional hazards model with adjustments for factors with a P value <0.1 in the univariate analysis. Results were summarized as hazard ratio (HR) and 95% confidence interval (CI). All probability values were two-sided, and a P value <0.05 was considered statistically significant. All statistical analyses were performed using JMP 7.0 (SAS Institute, Cary, North Carolina, USA).
Results
Patients’ demographic and surgical data
The baseline characteristics of the patients and surgical data are summarized in Table 1. The median patient age was 65 years (IQR, 58–71 years), and 83% of patients were men. The prevalence of patients who were dependent on inotrope agent prior to the surgery was 7.8%. The median values for LV end-diastolic and systolic dimension were 68 mm (IQR, 63–73 mm) and 57 mm (IQR, 53–64 mm), respectively; the LV ejection fraction was 27% (IQR, 22–34%). The median value for LA dimension was 50 mm (IQR, 33–69 mm), and the Doppler-derived systolic PAP was 53±15 mmHg (range, 30–120 mmHg).
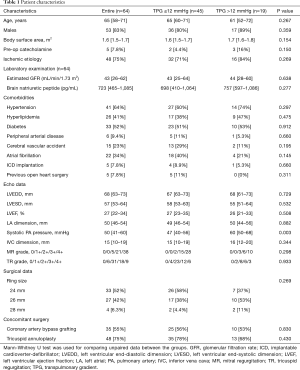
Full table
Prior to RMA, 45 patients had a TPG ≤12 mmHg (low TPG group), while 19 had a TPG >12 mmHg (elevated TPG group). The baseline characteristics according to the preoperative TPG are presented in Table 1. There were no significant differences in age, sex, heart failure severity, heart failure etiology, severity of comorbidities, LV function parameters, and severity of valvular disease between patients in both TPG groups; however, higher Doppler-derived systolic PA pressures were seen in patients from the elevated TPG group. In addition, there were no significant differences in the surgical data between both groups.
Changes in pulmonary vascular hemodynamics after RMA
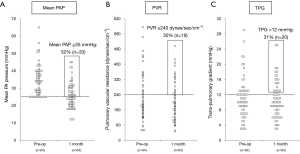
The changes in pulmonary vascular hemodynamics after RMA in the entire cohort are summarized in Table 2 and Figure 1. For the entire cohort (n=64), the mean PAP significantly decreased 1 month after surgery but remained abnormal (≥25 mmHg) in 33 patients (52%) (Figure 1A). PVR changed from 192 dynes/sec/cm−5 to 182 dynes/sec/cm−5; however, it remained abnormal (≥240 dynes/sec/cm−5) in 19 patients (30%) (Figure 1B). TPG did not significantly change, remaining abnormal (>12 mmHg) in 20 patients (31%) (Figure 1C).
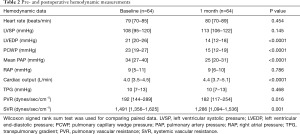
Full table
When comparing the changes in pulmonary hemodynamics between the two groups, the degree of improvements in the mean PAP, TPG, and PVR values were substantially smaller in patients with an elevated TPG (interaction effect P=0.015, 0.059, and 0.061, respectively). Consequently, patients with a preoperative TPG >12 mmHg were more likely to have postoperative mean PAP ≥25 mmHg (84% vs. 38%), TPG >12 mmHg (79% vs. 11%), and PVR ≥240 dynes/sec/cm−5 (84% vs. 6.7%) compared to patients with a preoperative TPG ≤12 mmHg (all P<0.001) (Table 3 and Figure 2). These findings indicate that the reversibility of PH secondary to chronic LV failure was limited to patients with an elevated preoperative TPG.
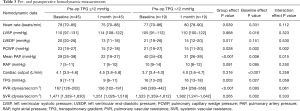
Full table
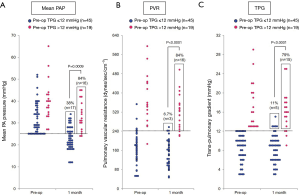
PCWP significantly decreased but remained abnormal (>15 mmHg) in 28 patients (44%). Patients with an elevated TPG showed lower degrees of decreases in LV end-diastolic pressure and PCWP than patients with a low TPG.
Serial echocardiographic data
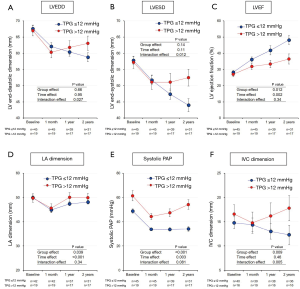
LV dimension decreased while LV ejection fraction increased in both groups 1 month after surgery, with subsequent changes showing distinctive characteristics (Figure 3A,B,C). These improvements were sustained for up to two years after surgery in patients in the low TPG group, whereas patients in the elevated TPG group showed gradual re-enlargement of LV dimensions as well as less improvement in the LV ejection fraction. LA dimension also significantly decreased 1 month after surgery; this then showed an increase in both groups, with higher values for patients in the elevated TPG group (Figure 3D). Both groups also exhibited a decrease in systolic PAP and IVC dimension 1 month after the surgery, with subsequent changes in each group showing distinctive characteristics. The values then remained stable or further decreased in the low TPG group whereas they steadily increased in the elevated TPG group (Figure 3E,F).
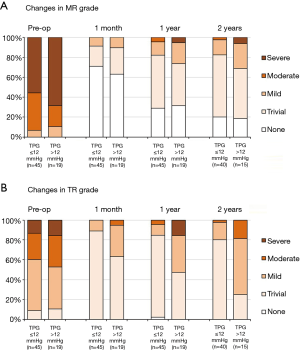
In both groups, mitral regurgitation grade substantially decreased after surgery, with a comparable prevalence of mitral regurgitation greater than or equal to the moderate grade at the two-year follow-up examination (31% vs. 18%, P=0.257) (Figure 4A). Tricuspid regurgitation grade in both groups also substantially decreased 1 month after the surgery, and the improvement was sustained for up to two years in patients in the low TPG group. Conversely, the improvement was limited in the elevated TPG group, with a greater prevalence of tricuspid regurgitation greater than or equal to the moderate grade at the two-year follow-up examination (75% vs. 20%, P<0.001) (Figure 4B).
Correlation between Doppler-derived systolic PAP and other echocardiographic parameters
Doppler-derived systolic PAP two years after surgery positively correlated with the corresponding end-diastolic (rho =0.429, P=0.005) and end-systolic (rho =0.449, P=0.003) LV dimensions, LA dimension (rho =0.483, P=0.001), tricuspid regurgitation grade (rho =0.611, P<0.001), and IVC dimension (rho =0.558, P<0.001), while a negative correlation was observed in the LV ejection fraction (rho =−0.390, P=0.012). There was also a notable yet weak correlation between systolic PAP and MR grade 2 years after surgery (rho =0.290, P=0.070).
Late clinical outcomes
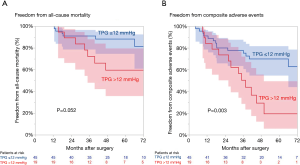
Clinical follow-up examinations were completed for all patients, with a mean duration of 54±27 months (range, 13–144 months). During the follow-up period, there were 16 mortalities and 24 unscheduled heart failure readmissions. Patients with an elevated TPG were more likely to experience mortality (9.6%/patient-year vs. 3.9%/patient-year, P=0.052) and readmission for heart failure (14%/patient-year vs. 5.9%/patient-year, P=0.006) than patients with a low TPG. Therefore, patients with an elevated TPG had a substantially lower survival rate (5-year, 60% vs. 88%, P=0.052) and freedom from composite adverse events (5 years, 20% vs. 70%, P=0.003) (Figure 5).
Predictors for adverse events after RMA
Among the examined baseline characteristics, preoperative catecholamine use, preoperative implantable cardioverter-defibrillator (ICD) implantation, Doppler-derived systolic PAP, catheter-measured left ventricular end-diastolic dimension (LVEDP), mean PAP, TPG, and PVR were identified to be associated with composite adverse events. Multivariate analysis revealed that a TPG >12 mmHg (adjusted HR 2.9, 95% CI: 1.2–6.9, P=0.017) and preoperative ICD implantation (adjusted HR 3.6, 95% CI: 1.0–13, P=0.044) were independent risk factors for late adverse events.
Discussion
The major findings of our study were as follows: (I) Mean PAP and PVR decreased, whereas TPG did not significantly change after RMA, and all measured variables remained abnormal in a noticeable number of patients despite successful MR reduction; (II) patients with a preoperative TPG >12 mmHg were more likely to show abnormal pulmonary hemodynamics and less improvement in LV function over time after RMA than patients with a TPG ≤12 mmHg; and (III) preoperative elevated TPG was significantly associated with an increased risk of mortality and/or unscheduled heart failure readmission after RMA.
Impact of RMA procedure on early postoperative pulmonary hemodynamics
Although several studies have described the reversibility of secondary PH after heart transplantation and/or LV assist device (LVAD) implantation for advanced heart failure, few have assessed pulmonary hemodynamic changes following RMA in patients with advanced cardiomyopathy (19,20). Alba et al. reported that pre-transplant LVAD therapy successfully decreased PH by significantly unloading the LV, even in patients with “fixed” PH (20). Meanwhile, Goland et al. found significant decreases in PVR and TPG, along with increased cardiac output 1 month after heart transplantation, with continued reductions after one year (21). In contrast to this, our study showed a modest decrease in PVR without significant change in TPG 1 month after RMA, followed by progressive worsening of PH in a subset of patients. These contrasting results could be explained by differences in the postoperative LV function between patients who underwent heart transplantation and patients who underwent RMA; the former seemed to have proper LV function with favorable postoperative LV filling, resulting in a significantly reversed passive or reactive PH component, whereas the latter showed partial LV reverse remodeling and continued abnormal LV filling pressure, accounting for unsolved reactive PH. These findings suggest that RMA may limit the reversibility of PH and vascular disease secondary to longstanding chronic LV failure, particularly in patients with an elevated TPG level at baseline.
Mechanism of PH progression and its impact on right ventricular function after RMA
Given that an increased TPG level reflects reactive pulmonary arterial vasoconstriction and/or pulmonary vascular remodeling, it was not surprising to note postoperative PH progression in patients with an elevated preoperative TPG level despite significant improvement in the MR grade. The noted significant difference in the postoperative trend of Doppler-derived systolic PAP in patients with and without elevated TPG might suggest that functional MR is not the sole factor for worsened pulmonary hemodynamics, since the degree of improvement in the MR grade for both groups after surgery was equivalent. PH progression observed in our patients with an elevated TPG could be due to further development of pulmonary vascular disease as well as long-standing elevated LV filling pressure, as predicted by fewer improvements in LV systolic function and a larger LA dimension observed using serial echocardiography. This speculation might be supported by the stronger correlation between systolic PAP with LV end-diastolic and end-systolic dimensions, LV ejection fraction, and LA dimension compared to the correlation between systolic PAP and MR grade. Furthermore, the above-mentioned speculation is consistent with our previous finding of a strong positive correlation between postoperative (1 month) catheter-measured pulmonary pressure and corresponding LVEDP in patients who underwent RMA (9).
Elevated pulmonary pressure and PVR represent an increase in both resistive and pulsatile right ventricular afterload, resulting in dilatation and maladaptive remodeling of the right heart chambers, functional tricuspid regurgitation, ultimately resulting in right ventricle dysfunction and failure (7,10,15). Despite the lack of findings regarding right ventricular dimensions, our results showing the gradual deterioration of functional tricuspid regurgitation and larger IVC dimension in association with an elevated TPG led us to speculate that these patients suffered from right ventricular dysfunction in association with PH progression to a certain extent; however, it is likely that patients with secondary PH acclimated to the increased afterload of elevated PAP. Altogether, these findings suggest that surgical intervention should be indicated for an optimal outcome before a pulmonary vascular disease becomes irreversible.
Limitations
This study was retrospective in nature, and the number of patients enrolled was small; thus, the conclusions drawn from the findings are limited. Also, the results shown in this study might have been biased due to the exclusion of 67 patients whose postoperative hemodynamic data were not available. The inclusion of patients with different etiologies for heart failure as well as those who underwent concomitant surgical intervention might have influenced the results. However, such concomitant procedures are usually required for patients with similar clinical and pathophysiologic situations, regardless of LV dysfunction etiology. In addition, the degree of pulmonary vascular remodeling in our patients could not be determined accurately because of the lack of pathological assessment of pulmonary arteries.
Conclusions
In summary, the reversibility of PH and vascular disease secondary to longstanding chronic LV failure may be limited in patients with pre-existing PH, particularly in patients with an elevated TPG at baseline. These findings suggest that the assessment of TPG should be included in post-RMA risk stratification.
Acknowledgments
Funding: This work was partially supported by the Takeda Science Foundation (Chuo-ku, Osaka, Japan). This research was partially supported by research funds to promote the hospital function of the Japan Labor Health and Welfare Organization. The authors thank Ms. Mariko Yamashita, Chikako Matsuo, and Misa Fujioka for their great help with clinical data collection.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-2898
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-2898
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-2898). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Osaka University (IRB number 08218-6), and the need for written informed consent from the patients was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Trichon BH, Felker GM, Shaw LK, et al. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am J Cardiol 2003;91:538-43. [Crossref] [PubMed]
- Carabello BA. The current therapy for mitral regurgitation. J Am Coll Cardiol 2008;52:319-26. [Crossref] [PubMed]
- Kainuma S, Toda K, Miyagawa S, et al. Restrictive mitral annuloplasty with or without coronary artery bypass grafting in ischemic mitral regurgitation. ESC Heart Fail 2020;7:1560-70. [Crossref] [PubMed]
- Kainuma S, Funatsu T, Kondoh H, et al. Beneficial effects of restrictive annuloplasty on subvalvular geometry in patients with functional mitral regurgitation and advanced cardiomyopathy. J Thorac Cardiovasc Surg 2018;156:630-8.e1. [Crossref] [PubMed]
- Kainuma S, Taniguchi K, Toda K, et al. B-type natriuretic peptide response and reverse left ventricular remodeling after surgical correction of functional mitral regurgitation in patients with advanced cardiomyopathy. J Cardiol 2015;66:279-85. [Crossref] [PubMed]
- Kainuma S, Taniguchi K, Toda K, et al. Restrictive mitral annuloplasty for functional mitral regurgitation: acute hemodynamics and serial echocardiography. Circ J 2011;75:571-9. [Crossref] [PubMed]
- Abramson SV, Burke JF, Kelly JJ Jr, et al. Pulmonary hypertension predicts mortality and morbidity in patients with dilated cardiomyopathy. Ann Intern Med 1992;116:888-95. [Crossref] [PubMed]
- Kainuma S, Taniguchi K, Toda K, et al. Pulmonary hypertension predicts adverse cardiac events after restrictive mitral annuloplasty for severe functional mitral regurgitation. J Thorac Cardiovasc Surg 2011;142:783-92. [Crossref] [PubMed]
- Kainuma S, Taniguchi K, Daimon T, et al. Does stringent restrictive annuloplasty for functional mitral regurgitation cause functional mitral stenosis and pulmonary hypertension? Circulation 2011;124:S97-106. [Crossref] [PubMed]
- Costard-Jäckle A, Fowler MB. Influence of preoperative pulmonary artery pressure on mortality after heart transplantation: testing of potential reversibility of pulmonary hypertension with nitroprusside is useful in defining a high risk group. J Am Coll Cardiol 1992;19:48-54. [Crossref] [PubMed]
- Rosenkranz S, Gibbs JS, Wachter R, et al. Left ventricular heart failure and pulmonary hypertension. Eur Heart J 2016;37:942-54. [Crossref] [PubMed]
- Guazzi M, Arena R. Pulmonary hypertension with left-sided heart disease. Nat Rev Cardiol 2010;7:648-59. [Crossref] [PubMed]
- Tumminello G, Lancellotti P, Lempereur M, et al. Determinants of pulmonary artery hypertension at rest and during exercise in patients with heart failure. Eur Heart J 2007;28:569-74. [Crossref] [PubMed]
- Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2009;54:S43-54. [Crossref] [PubMed]
- Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009;30:2493-537. [Crossref] [PubMed]
- Chatterjee NA, Upadhyay GA, Singal G, et al. Pre-capillary pulmonary hypertension and right ventricular dilation predict clinical outcome in cardiac resynchronization therapy. JACC Heart Fail 2014;2:230-7. [Crossref] [PubMed]
- Erickson KW, Costanzo-Nordin MR, O’Sullivan EJ, et al. Influence of preoperative transpulmonary gradient on late mortality after orthotopic heart transplantation. J Heart Transplant 1990;9:526-37. [PubMed]
- Murali S, Kormos RL, Uretsky BF, et al. Preoperative pulmonary hemodynamics and early mortality after orthotopic cardiac transplantation: the Pittsburgh experience. Am Heart J 1993;126:896-904. [Crossref] [PubMed]
- Goldstone AB, Chikwe J, Pinney SP, et al. Incidence, epidemiology, and prognosis of residual pulmonary hypertension after mitral valve repair for degenerative mitral regurgitation. Am J Cardiol 2011;107:755-60. [Crossref] [PubMed]
- Alba AC, Rao V, Ross HJ, et al. Impact of fixed pulmonary hypertension on post-heart transplant outcomes in bridge-to-transplant patients. J Heart Lung Transplant 2010;29:1253-258. [Crossref] [PubMed]
- Goland S, Czer LS, Kass RM, et al. Pre-existing pulmonary hypertension in patients with end-stage heart failure: impact on clinical outcome and hemodynamic follow-up after orthotopic heart transplantation. J Heart Lung Transplant 2007;26:312-18. [Crossref] [PubMed]


