
CD31+, CD38+, CD44+, and CD103+ lymphocytes in peripheral blood, bronchoalveolar lavage fluid and lung biopsy tissue in sarcoid patients and controls
Introduction
Sarcoidosis is a systemic inflammatory disease caused by the combined effects of genetic susceptibility and environmental exposures (1). Multiple potential infectious, non-infectious, organic, and inorganic environmental etiologic agents, unfortunately without any definitive demonstration of causality, have been proposed for sarcoidosis (2,3). It is likely that in predisposed individuals, sarcoidosis is the result of the interplay between different etiologic agents and the immune system (4).
Although the phenotypic expression of the disease appears to vary in different populations, in general, patients with sarcoidosis tend to improve over time (5). Many patients enter clinical remission within a few years of diagnosis; however, approximately one third experience chronic disease. Outcomes are generally less favourable for parenchymal pulmonary sarcoidosis compared to lymph node limited disease (6).
Established experimental sarcoidosis models are still lacking, while studies on pathophysiologic mechanisms as well as the search for diagnostic indicators are hampered by high variability in the course of the disease. One of the recent studies (7) using mass spectrometry based proteomics identified 4,306 proteins in BAL cells, of which 272 proteins were differentially expressed in sarcoidosis compared to controls, and 121 were differentially expressed comparing progressive vs. non-progressive sarcoidosis subjects.
The prognosis of sarcoidosis is highly disparate according to ethnic and genetic factors, the initial presentation, and other contributors (8). There is still no consensus on which tools are necessary to predict the progression of the disease or how to monitor its activity (9). If present during an early phase, ongoing inflammation and interstitial fibrosis, specific to the chronic phase of the disease, may alert the presence of a more serious form and, consequently, a tendicity to chronicity (10). Compared to patients with persistently active non-fibrotic disease, the potential complications and long-term outcomes are worse for those with fibrotic sarcoidosis (11). The extent of fibrosis on computed tomography is an independent predictor of mortality (12). Since the mechanisms driving the transition from inflammation to fibrosis in sarcoidosis are poorly understood (13) and the evolution and severity of the disease vary substantially, it is difficult to predict the disease course (14). It is not known if a fibrosis cascade is activated at disease onset or if it is a response to poorly controlled inflammation (11).
Some cytokines, chemokines as well as immune cells derived mediators were proposed to be useful for the diagnosis and follow-up of sarcoidosis patients; however, no single biomarker with proven unequivocal prognostic value has yet been identified. Up-to-know only one serum factor, angiotensin converting enzyme (ACE) is mentioned in International guidelines (15). Nevertheless its sensitivity and specificity are low (16,17), additionally, in the recent study (16) it was demonstrated that its clinical value as biomarker can be further reduced for patients treated with ACE inhibitors. Some other serum factors also have been proposed for sarcoidosis biomarkers, including activated macrophages and neutrophils secreted chitotriosidase, lysozyme, produced by monocyte-macrophage system and epithelioid cells and involved in granuloma formation, mucin protein Krebs von den Lungen-6 (17,18).
Bronchoalveolar lavage fluid examination is being used as diagnostic tool for forty years; best known indicators for sarcoidosis are lymphocytosis and an increased CD4+ to CD8+ lymphocyte ratio. BAL cell patterns and lymphocyte phenotyping often provide information useful for interstitial lung diseases differentiation (18), and most of the studies shows that CD4+/CD8+ ratio greater than 3.5 has high diagnostic specificity for lung sarcoidosis. However, its sensitivity is low, besides, specificity appears to be lower in advanced radiographic stages of the disease (19).
Consequently, there is still great interest in cellular biomarkers, which seem to be related to inflammation, immune cell migration, and fibrosis (20-22), including cell surface molecules or their ligands. We had performed literature search looking for a cellular markers that could help to predict the course of sarcoidosis and selected four molecules, e.g., CD31, CD38, CD44, and CD103. Many inflammatory cells and tissue cells with various, sometimes even opposite effects may be positive for CD31, CD38, CD44, and CD103 (23-29), but while sarcoidosis is driven by T-cell mechanisms, particularly the accumulation of activated CD4 T-cells in the lungs, allowing for T-cell attachment and transmigration through the endothelium (30-31), research was performed on T cell subpopulations CD4+ and CD8+.
CD31, also known as platelet endothelial cellular adhesion molecule-1 (PECAM-1), is an integral membrane protein expressed by endothelial cells, platelets, dendritic cells, and blood cells, including T lymphocytes. CD31 is known to be involved in leukocyte–leukocyte interactions, as well as in interactions between lymphocytes and the vascular endothelium and migration to the inflamed tissues through intercellular junctions (23,24). The ligand to the CD31 molecule is a transmembrane glycoprotein CD38, a multifunctional receptor and co-receptor involved in transmembrane signalling and cell adhesion, also contributing to modulation of antigen-mediated T-cell responses (25,32). The human CD38 molecule is expressed by immature hematopoietic cells, downregulated on mature cells, and re-expressed at high levels on activated lymphocytes, such as T cells, B cells, dendritic cells, and natural killer (NK) cells. CD38 ligation is followed by an increase in intracellular Ca2+, cell activation, proliferation, differentiation, and migration, as well as by the production and secretion of a panel of cytokines, such as interleukin (IL)-6, IL-10, interferon-γ, granulocyte macrophage colony stimulating factor (GM-CSF), and by proliferative effects (32). CD44 is a family of cell surface glycoproteins, also termed hyaladherin (HA), belonging to the group of cell adhesion molecules. HA is involved in cell-to-cell and cell-to-matrix interactions and participates in the regulation of hyaluronic metabolism, activation, and migration of lymphocytes, as well as the release of cytokines in areas of inflammation (28-30). CD44 participates in a wide variety of cellular functions, including lymphocyte activation, recirculation and homing. CD44 expression is known to be greater in the area of granuloma formation and fibrosis (33). One more biomarker, investigated in sarcoidosis patients is integrin αEβ7 (CD103), an adhesion molecule expressed on 95% of intraepithelial CD4+ lymphocytes in the mucosa, but on less than 2% of circulating peripheral blood lymphocytes (18). Differential expression of this marker on BAL CD4+ lymphocytes has been demonstrated, and CD103 has been proposed as a diagnostic marker of granulomatous lung disorders, such as sarcoidosis, albeit with controversial results.
The aim of the present prospective study was to examine immune cell profiles that could be associated with the processes and pathways underlying the pathophysiology of sarcoidosis. In this paper, we extended our previous study (31-38) through the prospective and simultaneous analysis of lymphocyte subsets in the blood, bronchoalveolar lavage fluid (BALF), and lung biopsy tissue of patients with newly diagnosed pulmonary sarcoidosis at the time of initial evaluation.
We present the following study in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-2396).
Methods
A total of 71 consecutive patients (25 were smokers and 46 were non-smokers) with newly diagnosed pulmonary sarcoidosis and 20 healthy controls (HCs) were enrolled in the study from 2013 to 2015 at the Center of Pulmonology and Allergology (Vilnius University Hospital Santaros Klinikos), and blood and BALF analyses were performed. Bronchoscopic lung biopsies were performed in 35 of these patients with sarcoidosis. In addition, 5 samples were taken from biopsies that exhibited no histological changes, and these were used as a control for the tissue analyses. All study patients were Caucasian. None of the patients (including controls) had any relevant medical history or comorbidity (e.g., tuberculosis) nor a history of exposure to organic or mineral dusts known to cause granulomatous lung disease, nor any immunosuppressive therapy—steroids, cytostatics, biotherapy.
Forty-four patients had non-Löfgren sarcoidosis, and the remaining 27 patients had Löfgren syndrome. Patients were grouped into stages according to chest radiography and high-resolution computed tomography (HRCT). Comparisons were made between the groups according to smoking history (25 patients) and the presence of Löfgren syndrome. All patients underwent diagnostic testing as part of the routine clinical investigation, including chest radiography, HRCT examination, pulmonary function test, fibreoptic bronchoscopy with bronchoalveolar lavage (BAL), bronchoscopic lung biopsy, and BALF cell and blood examination. All study tests were performed over two weeks, on average (in all cases, less than one month), from the first visit to our center. The diagnosis was confirmed according to the American Thoracic Society/European Respiratory Society/World Association for Sarcoidosis and other Granulomatous Disorders statement (15). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). A signed informed consent form was obtained from each participant. This study was approved by the Vilnius Regional Biomedical Research Ethics Committee (No. 158200-12-5591160).
Chest radiography and computed tomography
Chest radiographic staging was performed according to the Scadding criteria, as follows: stage 0, normal chest radiograph; stage I, mediastinal lymphadenopathy only; stage II, mediastinal lymphadenopathy with parenchymal lesion; stage III, parenchymal disease only; and stage IV, pulmonary fibrosis. HRCT scans were performed using 64-slice CT (GE LightSpeed VCT, GE Healthcare, Milwaukee, Wisconsin). Scan parameters were as follows: collimation of 64×1.25 mm; tube voltage, 120 kV; tube current, 660 mAs.
Fibreoptic bronchoscopy, bronchoalveolar lavage, and bronchoscopic lung biopsy
Fibreoptic bronchoscopy, BAL, and bronchoscopic lung biopsy were performed as described elsewhere (34,35). The subjects were premedicated with atropine, and lidocaine was delivered topically via an atomiser. The bronchoscope was inserted transnasally (in most cases) or orally and passed to segmental or subsegmental bronchus. BAL was performed in the right middle lobe, lingual, or in the area of greatest radiologic abnormality. Sterile isotonic saline at room temperature was instilled in two 50-mL aliquots (as standard procedure in our centre). Each aliquot was retrieved with gentle manual aspiration (usually 30–40 mL in total). The second aliquot was used to analyse BALF cells and lymphocyte subpopulations.
Bronchoscopic lung biopsy was performed under fluoroscopic guidance as follows: 6 to 10 pieces (3–4 mm2 each) of lung tissue were taken from suspicious areas for histopathological examination at the National Center of Pathology, Affiliate of Vilnius University Hospital Santaros Klinikos.
Immunostaining and flow cytometry of peripheral blood and bronchoalveolar lavage fluid samples
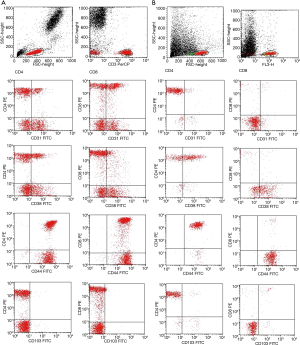
Peripheral blood and BALF samples were taken on the same day, and both were analysed at the Laboratory of Clinical Immunology and Blood Transfusion at the Center of Laboratory medicine (Vilnius University Hospital Santaros Klinikos). BALF for cell analysis was filtered through a 70 µm pore filter to remove mucus. The total cell numbers (Neubauer chamber) were determined, and the cellular material was sedimented by centrifugation (300 g for 7 min at 4 °C). Trypan blue dye (0.4%) was used for the assessment of BALF cell viability (the viability of the cells was 95%±5%). Cell differentials were obtained by counting at least 600 cells by light microscopy after staining with May-Grünwald-Giemsa stain (Merck, Darmstadt, Germany). The monoclonal antibodies (BD Biosciences, San Jose, CA, USA) used for lymphocyte characterisation were as follows: peridinin-chlorophyll-protein-complex (PerCP) conjugated anti-CD3; phycoerythrin (PE)-conjugated anti-CD4 and anti-CD8; and fluorescein-isothiocyanate (FITC)-conjugated anti-CD31, anti-CD38, anti-CD44, and anti-CD103. For both blood and BALF lymphocyte subpopulation analysis, 100 µL of the cell suspensions (1×106 cells) were incubated with monoclonal antibodies for 15 min: blood at room temperature, BALF at 4 °C in the dark. Afterwards, peripheral blood erythrocytes were lysed using 2 ml of lysing solution (incubation for 10 min at room temperature in the dark), and then the suspension was centrifuged at 300 g for 7 min at room temperature. The supernatant was discarded, and the remaining pellet washed by adding 2 ml of phosphate buffered saline (PBS) and centrifuging at 300 g for 7 min at room temperature (blood) or 4 °C (BALF). Afterwards, the supernatant was discarded, and the cells were re-suspended in 500 µL of 1% paraformaldehyde. Following cell fixation, flow cytometry was run on an FACS Calibur (BD Biosciences, San Jose, CA, USA). After proper instrument settings, calibration, and compensation, the results were analysed using CellQuestPro software (BD Biosciences, San Jose, CA, USA). Fixed cells were analysed within 24 hours. Cells were sequentially gated on lymphocytes [based on forward scatter (FSC) versus side scatter (SSC)] and on T lymphocytes (based on SSC versus CD3 PerCP histogram); after which, markers of interest on CD4 and CD8 subpopulations were determined. The isotype control γ1/γ2a/CD3 was used for the negative marker settings; these settings were applied for subsequent lymphocyte analysis with a minimum of 10,000 events. Peripheral blood and BALF flow cytometry dot plots of representative patient are shown in Figure 1.
Tissue preparation and digital image analysis of histology specimens
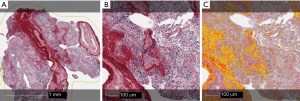
Biopsy specimens were fixed in formalin and stained with hematoxylin-eosin (HE) for histological investigation, and Picro-Sirius Red was used for fibrosis assessment. Immunohistochemistry was performed using formalin-fixed paraffin-embedded sections, which were cut 3 µm thick and mounted on positively charged slides (Figure 2). Immunohistochemistry for CD8+, CD38+, CD44+, and CD103+ on the whole tissue sections was performed using the Ultra View Universal DAB detection kit on a Ventana Benchmark Ultra staining system (Ventana Medical Systems, Tucson, Arizona, USA). Epitope retrieval was performed on the slides using Ventana Ultra CC1 buffer at 95 °C for 64 min. The sections were then incubated with DAKO anti-Human CD8+ mouse monoclonal antibody (C8/144B, 1:100 dilution), Cell Margue anti-CD38 rabbit monoclonal antibody (SP149, 1:100 dilution), Abcamanti-CD44 rabbit monoclonal: antibody (EPR1013Y, 1:100 dilution), and Abcamanti-Integrin alpha E rabbit monoclonal antibody (EPR4166, 1:500 dilution) at 37 °C for 32 min using the Ventana Ultraview DAB detection kit. Finally, the sections were developed in 3,3'-Diaminobenzidine as the chromogen at 37 °C for 8 min, counter stained with Mayer’s hematoxylin, and mounted.
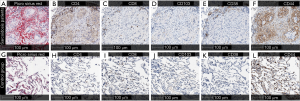
Immunohistochemistry for CD4+ was performed using the EnVision FLEX+ visualisation system on a DAKO Autostainer Link 48+ system (Agilent DAKO, USA). Epitope retrieval was performed on the slides using DAKO TRS High pH buffer at 97 °C for 20 min using the PT-link module (Agilent DAKO, USA). The sections were then incubated with DAKO anti-Human CD4+ mouse monoclonal antibody (4B12, 1:150 dilution) at 22 °C for 30 min using the EnVision FLEX+ detection kit, developed in DAB at 22 °C for 10 min, counter stained with Mayer’s hematoxylin, and mounted. Digital images were captured using the Aperio Scan-Scope XT Slide Scanner (Aperio Technologies, Vista, CA, USA) under 20× objective magnification (0.5 µm resolution).
Digital image analysis
Digital image analysis (DIA) was performed using the HALOTM Classifier Module and Cyto Nuclear v1.5 algorithms (IndicaLabs, NM, USA) with a manually selected region of interest (ROI) enclosing the tissue section. The software enables automated recognition of selected tissue areas and cell segmentation in scanned images. The nuclear/cytoplasmic analysis was calibrated to enumerate positive and negative inflammatory cell profiles in lung tissue. The positivity thresholds of DIA were monitored and individually calibrated by visual inspection in each immunohistochemical stain. The result of various inflammatory cell densities was calculated by cell counts in lung tissue per square millimetre. Fibrosis areas were calculated using the HALOTM Area Quantification v1.0 algorithm (Figure 3).
Statistical analysis
Statistical analysis was performed using SPSS software, Version 20.0 (Statistical Package for Social Sciences, IBM, USA). The mean, standard deviation (SD), and the available number of observations of the quantitative variables are presented. A non-parametric Kruskal Wallis criterion was used to evaluate all the parameters. Spearman’s rank correlation coefficient was used to determine the correlation. A P value of less than 0.05 was considered significant.
Results
The demographic characteristics of the patients, pulmonary function test values, Scadding radiological stages of studied groups as well as general characteristics of BALF cell counts and BALF lymphocyte subsets are shown in Table 2.
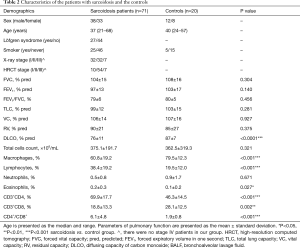
Full table
Blood vs. BALF lymphocyte subsets in the control and different radiographic stages of sarcoidosis
We compared control and sarcoidosis groups and no significant difference was found in the blood CD4+ T lymphocyte count (41.9%±7.3% vs. 41.1%±8.5%). However, the CD8+ T lymphocyte count in the sarcoidosis group was found to be significantly lower (32.0%±8.5% vs. 27.1%±9.0%, P<0.05). In BALF, on the contrary, significant differences were found (Figure 1).
Blood vs. BALF T lymphocyte subsets in different radiographic stages of the sarcoidosis and control group are represented in Figure 4 (CD4+ T cells) and Figure 5 (CD8+ T cells). The percentage of CD31+ and CD38+ peripheral blood cells of the control group was found to be significantly higher compared to BALF (P<0.0004) on both CD4+ and CD8+ T lymphocytes, whereas CD44+ was expressed equally on blood and BALF CD4+ T lymphocytes, but was significantly lower (P<0.02) on BALF CD8+ T cells. CD103+ cell percentage in the BALF was significantly higher compared to peripheral blood (P<0.00001 for the control group CD4+ and CD8+ T lymphocytes).
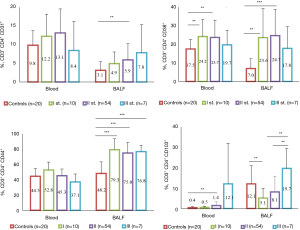
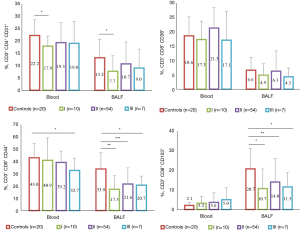
The percentage of studied marker in the blood was found to be sarcoidosis-stage-dependent. Specifically, CD4+CD38+ (P=0.004) and CD4+CD103+ (P=0.003) T-cell numbers were found to be significantly higher in radiographic stage II sarcoidosis patients compared with controls (Figure 4). The opposite was true for activation marker percentage on CD8+ T lymphocytes (Figure 5): CD44+ in the stage III group (P=0.035) was significantly lower than controls.
The percentages of several BALF lymphocyte subsets in sarcoid patients were significantly different from the controls. The percentages of BALF CD4+CD31+, CD4+CD38+, and CD4+CD44+ T cells were found to be significantly higher, while CD8+CD44+ T cells were significantly lower in the sarcoidosis group compared with control group. Some markers were found to be sarcoidosis-stage-dependent. Specifically, the expression of CD31 on CD4+ T cells was found to increase with the sarcoidosis stage (P=0.002 for stage II vs. the controls). CD4+CD38+ T cell numbers during stage I (P=0.001) and stage II (P<0.0001) were found to be significantly higher compared with controls and tended to decrease in more advanced stage III sarcoid patients. The CD44 marker had significantly higher expression on CD3+CD4+ cells of sarcoid patients of all three stages (P<0.0001). On the contrary, the number of CD4+CD103+ T cells was found to be significantly higher in the control compared with stage I sarcoid patients (12.6%±8.8% vs. 5.1%±4.8%, P=0.006) and tended to increase with the radiographic stage [8.1%±7.7% for stage II (P=0.028) 19.7%±9.7% for stage III, P=0.054]. For CD8+ T cells, no clear tendency in its CD38+ percentage changes could be found, while CD31+, CD44+ and CD103+ was significantly lower in sarcoid patients (P=0.035 for CD31+, P=0.001 for CD44+ and P=0.011 for CD103+ comparing stage I sarcoid patients with the controls).
We also performed a separate analysis of blood and BALF lymphocyte subsets in acute sarcoidosis (Löfgren syndrome) versus non-Löfgren syndrome patients and non-smoker patients (presented in Figure 6) versus patients who smoke (Figure 7).
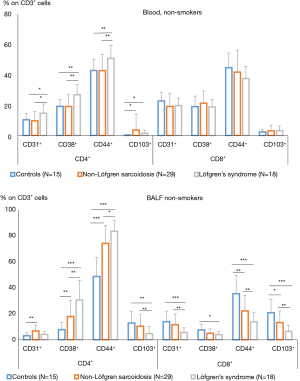
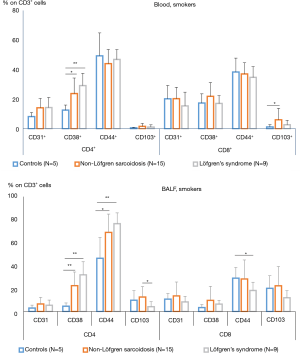
CD4+CD38+ expression on blood T-cells seems to be the only parameter that depends on smoking status, while comparing control groups significantly more positive lymphocytes was found in non-smokers (19.2%±4.5% vs. 12.6%±3.4%, P<0.008). In BALF, the non-smoker control group had a significantly higher CD8+CD38+ lymphocyte percentage than smokers (7.6%±4.5% vs. 3.4%±2.7%, P<0.03).
Blood vs. BALF lymphocyte subsets in the non-Löfgren syndrome and control groups compared with non-smokers and smokers
We compared non-smoking and non-Löfgren syndrome patients with controls and only percentage of CD4+CD103+ cells was significantly higher in non-Löfgren group compared with controls, 3.6%±10.5% versus 0.3%±0.4%, P=0.03. In the relevant groups of smokers, significantly more CD4+CD38+ marker positive cells were found in sarcoid patients, 23.8%±10.5% versus 12.6%±3.4% (P=0.025); and CD8+CD103+, 6.1%±7.7% versus 1.2%±1.6% (P=0.033). In BALF, differences between non-Löfgren syndrome and control groups were significantly more pronounced. For non-smokers, CD4+CD31+ was 6.8%±4.6% in the non-Löfgren group and 3.2%±2.3% in the control group (P=0.007), respectively CD4+CD38+ was 17.9%±12.6% versus 7.8%±5.6% (P=0.001) and CD4+CD44+, 74.2%±13.7% versus 48.8±14.8% (P<0.0001). The only percentage with no significant difference was CD4+CD103+. On the BALF CD8+ T-cells, on the contrary, the CD103 marker was much more highly expressed in the non-smoking control group (20.9%±10.4%) compared to the non-Löfgren non-smoking group (13.3%±8.9%, P=0.011).
The non-Löfgren group of smokers had significantly higher CD4+CD38+ percentage, in the BALF, reaching 22.5%±11.9% vs. 4.6%±2.99% in the control group of smokers (P=0.004), as well as CD4+CD44+, 68.9%±16.0% vs. 46.4%±18.1%, P=0.011.
Blood vs. BALF lymphocyte subsets in the Löfgren syndrome and control groups compared with non-smokers and smokers
Independent of smoking status, in the blood, patients with Löfgren syndrome had a higher percentage of CD4+CD31+ T-cells (14.7%±6.0% for non-smokers and 12.0%±6.8% for smokers) versus the controls [10.4%±4.1% (P=0.045) for non-smokers and 8.2%±2.8% (P=0.06) for smokers]. Difference was more pronounced for peripheral blood CD4+CD38+ percentage: the marker was expressed on 26.8%±6.7% non-smokers Löfgren syndrome patients T cells versus 19.2%±4.5% (P=0.001) for non-smoking control, and on 26.0%±8.2% smokers Löfgren syndrome patients lymphocytes versus 12.6%±3.4% (P=0.001) for smoking control. Expression of CD44 (50.8%±8.7% in the Löfgren syndrome group vs. 42.8%±7.4% in the control group, P=0.009) and of CD103 (1.4%±2.1% in the Löfgren syndrome group vs. 0.3%±0.4% in the control group, P=0.001) on CD4+ blood T lymphocytes differed in non-smokers only.
In BALF, differences were also much more pronounced in the non-smokers Löfgren syndrome group. CD38+ on CD4+ T cells was 30.6%±15.2% in the Löfgren syndrome group and 7.8%±5.6% in the control group (P=0.002), CD44+ was 83.5%±8.3% in Löfgren syndrome group vs. 48.8%±14.8% in control (P=0.011), and CD103+ was 4.8%±5.4% in Löfgren syndrome patients versus 12.9%±9.2% in control (P=0.002). On the BALF CD8+ T cells, all activity markers studied with the exception of CD8+CD38+ had significantly higher expression in the control non-smokers group compared to the Löfgren syndrome non-smokers group.
For smokers Löfgren group patients BALF CD4+CD38+ lymphocytes number significantly exceeded same population in smoking controls (45.0%±11.4% vs. 4.6%±2.9%, P=0.001). Also, the BALF CD4+CD44+ T cell count was found to be significantly higher in smokers Löfgren group patients compared to smoking control (87.0%±9.5% vs. 46.4%±18.1%, P=0.001).
Blood vs. BALF lymphocyte subsets in the non-Löfgren and the Löfgren groups compared with non-smokers and smokers
We compared patients from the non-Löfgren group and the Löfgren group, the impact of smoking status on the percentage markers studied was also clearly seen both in blood and the BALF. In the blood of non-smoking groups, most activity markers on CD4+ T cells differed significantly in the Löfgren syndrome group vs. the non-Löfgren syndrome patients (P=0.01 for CD4+CD31+, P=0.002 for CD4+CD38+, P=0.002 for CD4+CD44+). In the BALF some indices were higher in non-smoking Löfgren group patients, specifically CD4+CD38+ (30.6%±15.2% vs. 17.9%±12.6%, P=0.002) and CD4+CD44+ (83.5%±8.3% vs. 74.2%±13.7%, P=0.011). Nevertheless, in most cases percentages in the Löfgren group was significantly lower than in the non-Löfgren syndrome patients: CD4+CD103+ (4.8%±5.4% vs. 10.5%±8.8%, P=0.002), CD8+CD31+ (5.7%±3.6% vs. 11.6%±8.4%, P=0.007), CD8+CD44+ (13.8%±7.1% vs. 22.3%±11.9%, P=0.006), and CD8+CD103+ (13.3%±8.9% vs. 3.9%±2.4%, P=0.004).
Smoking had masked the differences mentioned above, but in spite of this, for smokers CD4+CD38+ and CD4+CD44+ were also higher in Löfgren group compared with non-Löfgren patients, while other markers (CD31 and CD103) were lower. A significant difference was found only for CD4+CD103+ T cells (3.0%±4.4% vs. 12.7%±9.1%, P=0.01).
Lung tissue lymphocyte subsets
The lymphocyte subsets and percentage of collagen in the lung tissue of sarcoidosis patients and controls are shown in Table 1. Surprisingly, few lymphocyte subsets were found to be significantly different in the sarcoidosis group compared with controls. Specifically, in the lung tissue, the percentage of CD4+ and CD103+ cells was found to be significantly higher and the percentage of CD44+ cells was lower (the patients with Löfgren syndrome were excluded from this analysis).
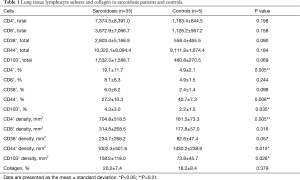
Full table
We have not found a clear relationship between lymphocyte subsets and the sarcoidosis stage (data not shown). Moreover, the analysis of the impact of smoking on lung tissue surprisingly revealed that only the percentage of collagen was decreased in smoking sarcoid patients compared with non-smokers (17.9±8.5 vs. 22.6±5.3, P=0.015).
Correlations between blood, BALF, and lung tissue lymphocyte subsets
Several significant correlations between blood, BALF, and lung tissue lymphocyte subsets and other indices were found (presented in Tables S1,S2,S3). Only the most important ones are presented here. Positive correlations between the percentage of blood CD3+CD4+CD38+ and BALF CD3+CD4+CD38+ (r=0.510, P=0.0001) and between the percentage of blood CD3+CD4+CD44+ and BALF CD3+CD4+CD44+ (r=0.362, P=0.002) were found. The percentage of blood CD3+CD8+CD103+ was positively correlated with the tissue total CD44+ cell count (r=0.632, P=0.0001).
Surprisingly, while comparing the corresponding BALF and lung tissue lymphocyte subsets, only BALF CD3+CD4+CD103+ correlated with the tissue total CD103+ cell count (r=0.473, P=0.020), CD103+ (%) cells (r=0.514, P=0.010), and CD103+ density (r=0.408, P=0.048).
Furthermore, we found weak but significant negative correlations between the percentage of blood CD3+CD4+CD103+ and the DLCO (r=−0.259, P=0.259) value and between the BALF CD3+CD4+CD31+ and total lung capacity (TLC) (r=−0.261, P=0.029) and vital capacity (VC) (r=−0.242, P=0.043). Moreover, we found significant negative correlations between the tissue CD44+ and CD103+ cells with several pulmonary function test indices, as follows: CD44+ total with forced vital capacity (FVC) (r=−0.406, P=0.044), TLC (r=−0.503, P=0.011) and VC (r=−0.406, P=0.044); CD44+ (%) with DLCO (r=−0.414, P=0.040); CD103+ total with FVC (r=−0.459, P=0.021), TLC (r=−0.415, P=0.039) and VC (r=−0.481, P=0.015).
Discussion
The most characteristic signs of sarcoidosis are granulomas, and the essential component of the immune response for granuloma formation is T cell activation and preferential homing of activated T cells to the tissue. In spite of long-lasting efforts of the scientific community, research in the determination of the causative agents of sarcoidosis is still lacking. Studies of the key cellular players are essential for the understanding the etiopathogenesis of this disease. The mechanisms driving the transition from inflammation to fibrosis are also poorly understood; although, two phases are apparent. The first is chronic inflammation and the second is fibrotic transformation. It is likely that chronic sarcoidosis may not simply be the persistence of acute sarcoidosis but a fundamentally distinct form of remitting disease from the very beginning (12).
We investigated 71 patients with newly diagnosed sarcoidosis. For the analysis, study data were grouped by radiologic disease stages, clinical manifestation, and smoking status.
In general, the majority of the investigated T lymphocyte subsets and activation marker expression profiles differed significantly between the sarcoidosis patients and the controls, especially in BALF. A marked difference in the percentage of lymphocyte subsets (in blood and in BALF) was found when comparing patients with and without Löfgren syndrome. Additionally, the percentage of lymphocyte subsets differed significantly in patients with different radiological stages of sarcoidosis (both in blood and in BALF). Smoking status also had considerable impact on the lymphocyte subset profiles of sarcoidosis patients.
The most interesting and promising results were obtained for the cell markers, represented by CD31+, CD38+, CD44+, and CD103+ expression on T lymphocytes and especially on CD4+ T cells. These results are: (I) increase of percentage of CD3+CD4+CD38+ both in BALF and blood, and increase of percentage of CD3+CD4+CD44+ in BALF in Löfgren syndrome patients comparing with patients without Löfgren syndrome (probable reflection of acute immune response), (II) increase of percentage of CD3+CD4+103+ T cells in BALF and in blood in patients without Löfgren syndrome (compared with Löfgren syndrome patients) and increase of percentage of CD3+CD4+103+ T cells both in BALF and in blood with increasing (i.e., more advanced) sarcoidosis stage (probable reflection of ongoing immune response), (III) percentage of BALF CD3+CD4+CD31+ is increased in sarcoidosis patients when compared with controls independently of presence of Löfgren syndrome, smoking status or stage of the disease (possible additional diagnostic marker).
CD31, is expressed in naïve recent thymic emigrants, but is downregulated after T-cell activation events and is absent from memory cells. In CD8+ T cells, CD31 expression is dynamically regulated, e.g., strongly downregulated during acute infection but re-expressed to intermediate levels in memory cells (26). CD31 is implicated in the development of atherosclerosis and its clinical complications (37). Nevertheless, papers describing CD31+ cells in sarcoidosis or expression on BALF cells in other respiratory diseases are still lacking. To our knowledge, only Ziora et al. (38) has analysed the serum concentrations of the soluble PECAM-1 molecule in the blood of sarcoidosis patients, reporting that PECAM-1 concentrations were similar in sarcoidosis patients and controls. In our study, we observed a very similar phenomenon, e.g., CD31 expression on the peripheral blood T-cell surface in sarcoid patients and controls was comparable.
While analysing CD31 expression on the immune cell surface we discovered that a much higher proportion of blood CD4+ and CD8+ T cells express this receptor compared to BALF. This difference is possible due to a higher number of BALF memory T lymphocytes, lacking CD31 molecule on their surface. Moreover, expression of CD31 on CD4+ T cells in BALF was found to increase with the sarcoidosis stage (p=0.002 for stage II vs. the control), while on CD8+ T lymphocytes, on the contrary, in stage I sarcoid patients significantly less CD31 positive cells (P=0.035) were found.
Ziora et al. demonstrated involvement of CD38 molecule in the early phases of lymphocyte binding to the endothelium through direct interaction with CD31 (26). CD38–CD31 interactions upregulate integrin expression and promote the ensuing steps in the adhesion cascade. The finding of selectin-like behaviour of the CD38 molecule resulted in identification of CD31 as an endothelial cell surface ligand (26). It was shown that CD38 regulates inflammation by modulating leukocyte responses and migration to sites of inflammation. It has been proposed as an early immune marker that reflects T-cell activation in allergies and some infectious diseases (25-27). CD38 is defined as both a cell surface enzyme (i.e., ectoenzyme) and as a receptor. The possible effect of CD38 receptor on fibrosis formation was indicated by El-Chemaly at al., showing that the blood CD38+ memory B cell count was significantly higher in patients with pulmonary fibrosis compared to unaffected controls (20). Furthermore, Lee et al. revealed a significantly increased proportion of activated CD38+ cells within the naïve B-cell compartment of severe chronic sarcoidosis patients in comparison with healthy controls (39). Nevertheless, until now, no data on T cell CD38 expression in sarcoidosis has been available.
The impact of this molecule on mucosal immunity was studied by Deaglio et al. on intestinal lamina propria colonising T lymphocytes. This research showed that virtually all CD31+ cells co-expressed CD38, whereas only ~50% of CD38+ cells were CD31+ (40). When comparing the values of CD31 and CD38 marker expression observed in our study, it can be assumed that the same is valid for BALF. Unfortunately, as well as we applied three-color cytometry, e.g., FITC conjugated monoclonal antibodies were used for both CD31 and CD38 molecules, it could not be directly proven. However, we noted many similarities in CD31 and CD38 expression both on the peripheral blood and BALF T cell surface. On peripheral blood CD4+ T cells, CD38 was also more highly expressed in stage II sarcoid patients compared with controls (P=0.004). Unlike CD31, the effect of smoking was not seen on the blood or BALF cells. For both smoking and non-smoking sarcoidosis patients, and especially the Löfgren syndrome group, patients’ CD3+CD4+ T cells had significantly higher CD38 expression compared with controls. Therefore, the increase in CD38 expression on CD4+ T lymphocytes may be associated with the acute inflammatory response in sarcoidosis.
Culty et al. had shown that CD44 expression is greater in the area of granuloma formation and fibrosis (33). We did not find significant differences when comparing blood CD44 expression on CD4+ T cells between the control group and the sarcoidosis group, only a tendency to increase of expression (P=0.056) between stage I sarcoid patients and controls. Meanwhile, on CD8+ T cells CD44 expression was found to be significantly lower (P=0.035) when comparing stage III and control groups. When analysing blood lymphocyte subsets, grouped by sarcoidosis activity, in non-smoking Löfgren syndrome patients significant activation of CD4+CD44+ expression was revealed (P=0.007) compared with controls. However, no difference was found in smokers or in the non-Löfgren group. Kasuga et al. evaluated soluble CD44 in the serum (sCD44) of 13 sarcoidosis patients and 56 controls, as well as in the BALF of 11 sarcoidosis patients and 10 controls (41). In patients with sarcoidosis, the serum CD44 level was significantly higher than that of controls. BALF sCD44 levels tended to be higher in sarcoidosis patients than in controls. Nevertheless, no statistically significant difference was recognised. We investigated the percentage of the CD44 cells, and in our study, the difference in the percentages of BALF lymphocyte subsets was found to be much more pronounced than in the blood. We had noticed significantly more CD44 positive CD4+ T cells in non-smokers Löfgren syndrome patients BALF, compared with non-Löfgren syndrome ones. Smoking masked this difference, but the trends remained the same. Kaiser et al. (42) reported contrary findings on CD44 expression. In this BALF study of four Löfgren syndrome patients (all HLA-DRB1*03 allele positive) and four non-Löfgren syndrome patients (all HLA-DRB1*03 allele negative), 33 markers, including CD44, were analysed by mass cytometry. Expression of the adhesion molecule CD44 was found to be significantly reduced in Löfgren syndrome CD4+ T cells compared to the background. Conversely, non-Löfgren syndrome cells showed a shift in CD44 expression of similar magnitude but opposite direction. The reasons for the discrepancy between the work by Kaiser et al. and our findings are not clear. The association between HLA and genetic background was not analysed in our study. Besides, in our study we had used different BALF preparation and cell enumeration method. Lately, knowledge on the genetic architecture of sarcoidosis has been evolving, showing genetic differences between Löfgren syndrome and non-Löfgren syndrome patients, but the epigenetics events that determine the course of the disease are not sufficiently known (43,44). Our findings on CD4+CD38+ and CD4+CD44+ T cells suggest that further investigations are required in order to assess these markers as indicators of inflammatory activity in sarcoidosis patients. Analysis of these markers during active episodes of sarcoidosis seems to be most promising.
Integrin CD103 was the only cell marker we chose that had possible diagnostic value in terms of its expression as an additional biomarker of sarcoidosis. It is known that this molecule can promote T-cell migration into the epithelium and is involved in the retention of lymphocytes in the mucosa. Constant CD103 expression can reflect antigen(-s) persistence in the lung tissue (22,45-48).
CD103 expression on control group peripheral blood T cells was very low compared to the other markers we studied. CD103 was the only marker, which expression in BALF was significantly higher compared to peripheral blood (P<0.00001 for both CD4+ and CD8+ T lymphocytes). CD103 molecule percentage on blood cells was found to be sarcoidosis-stage-dependent: the percentage of CD4+CD103 T cells in control was 12.6%±8.8%, significantly higher than in stage I sarcoid patients (5.1%±4.8%, P=0.006), and increased with the stages (8.1%±7.7% for stage II, 19.7%±9.7% for stage III). In BALF, this molecule expression was also dependent on sarcoidosis activity, being significantly lower in Löfgren syndrome patients compared with non-Löfgren syndrome patients. In line with these findings, the percentage and density of CD103+ positive cells in lung tissue were significantly higher in sarcoidosis patients (notably, non-Löfgren syndrome patients) compared with controls. Additionally, we found a negative correlation between the percentage of blood CD4+CD103+ T cells and the diffusing capacity for carbon monoxide (DLCO) value.
Our results on CD4+CD103+ T cells in BALF are in line with the study by Lohmeyer et al. (49). These authors found that CD103+ expression on BALF CD4+ T cells displayed subgroup dependency: proportions significantly lower than normal were noted in chest radiographic stage I, but increased proportions in stages II and III were found. The lowest expression was found in Löfgren syndrome patients. Other authors (50-55) have also revealed that BALF CD4+ T lymphocytes from sarcoidosis patients compared with other interstitial diseases have reduced CD103 expression.
Several explanations were proposed [discussed in (50)]. In brief, version is raised that in sarcoidosis CD4+ BALF lymphocytes originate not from the mucosal inductive sites, but from the peripheral blood. In other lung diseases a mucosal origin for BALF lymphocytes is suspected, with CD103+ lymphocytes relocating from mucosal areas into the lung and proliferating in response to local stimuli (50).
Braun et al. suggested that in patients with inflammatory lung diseases CD103-expressing CD4+ T cells in the lung are continuously activated, long-living cells (53). However, Braun et al. found no differences between the CD103+ and CD103– populations with respect to pro-inflammatory parameters. Based on their data, Heron et al. proposed that a higher proportion of CD4+CD103+ T cells might be associated with fibrosis formation in pulmonary sarcoidosis (54). Bretagne et al. found a significant negative correlation between the BALF CD103+CD4+/CD4+ ratio and FVC, TLC, and DLCO at last study visit. They suggested that the BALF CD103+CD4+/CD4+ ratio may be of interest as a prognostic marker in sarcoidosis (55).
Taking the data from this study and the reports of other authors together (53,54), it is reasonable to consider that CD4+CD103+ T cells in BALF and blood might be potential prognostic markers in sarcoidosis. At present, we speculate two possible scenarios: a relative deficit of CD4+CD103+ T cells in BALF reflects acute inflammation (immune response), or relative augmentation of CD4+CD103+ T cells in BALF reflects persistent inflammation (immune response) in the lungs. It seems reasonable to address this issue in future studies.
Like our previous preliminary findings (35,56) and the results of other authors (49), clinical manifestation, radiological sarcoidosis stage, and smoking status should always be taken into account when analysing lymphocyte subsets and activation marker expression. Which lymphocyte subpopulations are most important as prognostic factors and, maybe, additional diagnostic markers for sarcoidosis still need to be resolved in future studies.
Our study has strengths and limitations. The strength of our study is that it was carried out prospectively with the primary aim of investigating several biomarkers in newly diagnosed sarcoidosis patients. Secondly, we investigated lymphocyte subsets simultaneously in blood, BALF, and lung tissue. Thirdly, for enumeration of lung tissue cells we applied DIA. Moreover, the study population was fairly large, and the study was performed in a specialised tertiary healthcare facility.
The limitations of the study are related to the unavailability of lung biopsy data in the patients with Löfgren syndrome (due to the study design). It was not possible to analyse lymphocyte subsets in lung tissue for this group of patients. Also, lung tissue cells were analysed by single immunohistochemistry, without the possibility to obtain multiplex data on the lymphocyte subpopulations and other lung tissue cells which should be removed from final analysis. In contrast, in blood and BALF, we used triple staining with monoclonal antibodies. Another limitation is that this was a single-centre study (this reflects the patient population of our country since our centre is the primary centre for interstitial lung disease). Therefore, these results may reflect the sarcoidosis manifestation of our patient population.
Conclusions
We found that lymphocyte subtypes in blood, BALF, and lung tissue were substantially different in sarcoidosis patients at the time of diagnosis compared with healthy persons. Furthermore, the lymphocyte subtype profiles were significantly associated with the clinical manifestation, radiological sarcoidosis stage, and smoking status. The lymphocyte profiles in BALF and lung tissue did not correlate, which may indicate the need for assessment of both profiles in further studies. Increase of CD3+CD4+CD31+ in BALF may serve as supporting evidence for a diagnosis of sarcoidosis. Increase of CD3+CD4+CD38+ in BALF and blood and CD3+CD4+CD44+ in BALF—are markers of acute immune response in sarcoidosis. CD3+CD4+103+ T cells in BALF and in blood are markers of a persistent immune response in sarcoidosis patients and are a probable prognostic factor of the chronic course of the disease.
Acknowledgments
Funding: The study was funded by the EU structural support project No VP1-3.1-ŠMM-01-V-03-002, (2011–2014): Improvement of Training of High Qualification Specialists Conformed to the State and Society Needs in Biomedical field (BIOMEDOKT).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-2396
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-2396
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-20-2396
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-2396). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Vilnius Regional Biomedical Research Ethics Committee (No. 158200-12-5591160), and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen ES. Innate immunity in sarcoidosis pathobiology. Curr Opin Pulm Med 2016;22:469-75. [Crossref] [PubMed]
- Saidha S, Sotirchos ES, Eckstein C. Etiology of sarcoidosis: Does infection play a role? Yale J Biol Med 2012;85:133-41. [PubMed]
- Celada LJ, Hawkins C, Drake WP. The etiologic role of infectious antigens in sarcoidosis pathogenesis. Clin Chest Med 2015;36:561-8. [Crossref] [PubMed]
- Beijer E, Veltkamp M, Meek B, et al. Etiology and immunopathogenesis of sarcoidosis: Novel insights. Semin Respir Crit Care Med 2017;38:404-16. [Crossref] [PubMed]
- Judson MA, Boan AD, Lackland DT. The clinical course of sarcoidosis: Presentation, diagnosis, and treatment in a large white and black cohort in the United States. Sarcoidosis Vasc Diffuse Lung Dis 2012;29:119-27. [PubMed]
- Patterson KC, Chen ES. The pathogenesis of pulmonary sarcoidosis and implications for treatment. Chest 2018;153:1432-42. [Crossref] [PubMed]
- Bhargava M, Viken KJ, Barkes B, et al. Novel protein pathways in development and progression of pulmonary sarcoidosis. Sci Rep 2020;10:13282. [Crossref] [PubMed]
- Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: Comparison of survival with the general population and causes of death. Eur Respir J 2011;38:1368-73. [Crossref] [PubMed]
- Llabres M, Brito-Zerón P, Ramos-Casals M, et al. Synthetic pharmacotherapy for pulmonary sarcoidosis. Expert Opin Pharmacother 2019;20:1397-404. [Crossref] [PubMed]
- Silva AL, Melo N, Caetano Mota P, et al. Pulmonary sarcoidosis: Prognostic factors at diagnosis in patients from North of Portugal. Reumatol Clin 2020;16:468-72. [Crossref] [PubMed]
- Patterson KC, Strek ME. Pulmonary fibrosis in sarcoidosis. Clinical features and outcomes. Ann Am Thorac Soc 2013;10:362-70. [Crossref] [PubMed]
- Kirkil G, Lower EE, Baughman RP. Predictors of mortality in pulmonary sarcoidosis. Chest 2018;153:105-13. [Crossref] [PubMed]
- Bonham CA, Strek ME, Patterson KC. From granuloma to fibrosis: Sarcoidosis associated pulmonary fibrosis. Curr Opin Pulm Med 2016;22:484-91. [Crossref] [PubMed]
- Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet 2014;383:1155-67. [Crossref] [PubMed]
- Costabel U, Hunninghake GW. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J 1999;14:735-7. [Crossref] [PubMed]
- d'Alessandro M, Bergantini L, Perrone A, et al. Serial investigation of angiotensin-converting enzyme in sarcoidosis patients treated with angiotensin-converting enzyme inhibitor. Eur J Intern Med 2020;78:58-62. [Crossref] [PubMed]
- Bergantini L, Bianchi F, Cameli P, et al. Prognostic biomarkers of sarcoidosis: a comparative study of serum chitotriosidase, ACE, lysozyme, and KL-6. Dis Markers 2019;2019:8565423 [Crossref] [PubMed]
- d'Alessandro M, Carleo A, Cameli P, et al. BAL biomarkers' panel for differential diagnosis of interstitial lung diseases. Clin Exp Med 2020;20:207-16. [Crossref] [PubMed]
- Spagnolo P, Rossi G, Trisolini R, et al. Pulmonary sarcoidosis. Lancet Respir Med 2018;6:389-402. [Crossref] [PubMed]
- El-Chemaly S, Cheung F, Kotliarov Y, et al. The immunome in two inherited forms of pulmonary fibrosis. Front Immunol 2018;9:76. [Crossref] [PubMed]
- Johnson P, Arif AA, Lee-Sayer SSM, et al. Hyaluronan and its interactions with immune cells in the healthy and inflamed lung. Front Immunol 2018;9:2787. [Crossref] [PubMed]
- McMaster SR, Wein AN, Dunbar PR, et al. Pulmonary antigen encounter regulates the establishment of tissue-resident CD8 memory T cells in the lung airways and parenchyma. Mucosal Immunol 2018;11:1071-8. [Crossref] [PubMed]
- Schenkel AR, Chew TW, Chlipala E, et al. Different susceptibilities of PECAM-deficient mouse strains to spontaneous idiopathic pneumonitis. Exp Mol Pathol 2006;81:23-30. [Crossref] [PubMed]
- Lishnevsky M, Young LC, Woods SJ, et al. Microhemorrhage is an early event in the pulmonary fibrotic disease of PECAM-1 deficient FVB/n mice. Exp Mol Pathol 2014;97:128-36. [Crossref] [PubMed]
- Gally F, Hartney JM, Janssen WJ, et al. CD38 plays a dual role in allergen-induced airway hyperresponsiveness. Am J Respir Cell Mol Biol 2009;40:433-42. [Crossref] [PubMed]
- Guedes AG, Jude JA, Paulin J, et al. Airway responsiveness in CD38-deficient mice in allergic airway disease: studies with bone marrow chimeras. Am J Physiol Lung Cell Mol Physiol 2015;308:L485-93. [Crossref] [PubMed]
- Adekambi T, Ibegbu CC, Cagle S, et al. Biomarkers on patient T cells diagnose active tuberculosis and monitor treatment response. J Clin Invest 2015;125:1827-38. [Crossref] [PubMed]
- Shimizu Y, Van Seventer GA, Siraganian R, et al. Dual role of the CD44 molecule in T cell adhesion and activation. J Immunol 1989;143:2457-63. [PubMed]
- Goodison S, Urquidi V, Tarin D. CD44 cell adhesion molecules. Mol Pathol 1999;52:189-96. [Crossref] [PubMed]
- Rivera NV, Hagemann-Jensen M, Ferreira MAR, et al. Common variants of T-cells contribute differently to phenotypic variation in sarcoidosis. Sci Rep 2017;7:5623. [Crossref] [PubMed]
- Aleksonienė R, Zeleckienė I, Matačiūnas M, et al. Relationship between radiologic patterns, pulmonary function values and bronchoalveolar lavage fluid cells in newly diagnosed sarcoidosis. J Thorac Dis 2017;9:88-95. [Crossref] [PubMed]
- Deaglio S, Aydin S, Vaisitti T, et al. CD38 at the junction between prognostic marker and therapeutic target. Trends Mol Med 2008;14:210-8. [Crossref] [PubMed]
- Culty M, O’Mara TE, Underhill CB, et al. Hyaluronan receptor (CD44) expression and function in human peripheral blood monocytes and alveolar macrophages. J Leukoc Biol 1994;56:605-11. [Crossref] [PubMed]
- Danila E, Žurauskas E, Loskutovienė G, et al. Significance of bronchoscopic lung biopsy in clinical practice. Adv Med Sci 2008;53:11-16. [Crossref] [PubMed]
- Danila E, Norkūnienė J, Jurgauskienė L, et al. Diagnostic role of BAL fluid CD4/CD8 ratio in different radiographic and clinical forms of pulmonary sarcoidosis. Clin Respir J 2009;3:214-21. [Crossref] [PubMed]
- Newman DK, Fu G, McOlash L, et al. PECAM-1 (CD31) expression in naïve and memory, but not acutely activated, CD8+ T cells. J Leukoc Biol 2018;104:883-93. [Crossref] [PubMed]
- Marelli-Berg FM, Clement M, Mauro C, et al. An immunologist’s guide to CD31 function in T-cells. J Cell Sci 2013;126:2343-52. [Crossref] [PubMed]
- Ziora D, Jastrzębski D, Adamek M, et al. Circulating concentration of markers of angiogenic activity in patients with sarcoidosis and idiopathic pulmonary fibrosis. BMC Pulm Med 2015;15:113. [Crossref] [PubMed]
- Lee NS, Barber L, Akula SM, et al. Disturbed homeostasis and multiple signalling defects in the peripheral blood B-cell compartment of patients with severe chronic sarcoidosis. Clin Vaccine Immunol 2011;18:1306-16. [Crossref] [PubMed]
- Deaglio S, Mallone R, Baj G, et al. Human CD38 and its ligand CD31 define a unique lamina propria T lymphocyte signaling pathway. FASEB J 2001;15:580-2. [Crossref] [PubMed]
- Kasuga I, Minemura K, Nasu H, et al. Elevated serum soluble CD44 level in sarcoidosis. Int J Mol Med 2000;6:679-82. [Crossref] [PubMed]
- Kaiser Y, Lakshmikanth T, Chen Y, et al. Mass cytometry identifies distinct lung CD4+ T cell patterns in Löfgren’s syndrome and non-Löfgren’s syndrome sarcoidosis. Front Immunol 2017;8:1130. [Crossref] [PubMed]
- Rivera NV, Ronninger M, Shchetynsky K, et al. High-density genetic mapping identifies new susceptibility variants in sarcoidosis phenotypes and shows genomic-driven phenotypic differences. Am J Respir Crit Care Med 2016;193:1008-22. [Crossref] [PubMed]
- Kaiser Y, Eklund A, Grunewald J. Moving target: Shifting the focus to pulmonary sarcoidosis as an autoimmune spectrum disorder. Eur Respir J 2019;54:1802153 [Crossref] [PubMed]
- Sung SS, Fu SM, Rose CE Jr, et al. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol 2006;176:2161-72. [Crossref] [PubMed]
- Beauchamp NM, Yammani RD, Alexander-Miller MA. CD8 marks a subpopulation of lung-derived dendritic cells with differential responsiveness to viral infection and toll-like receptor stimulation. J Virol 2012;86:10640-50. [Crossref] [PubMed]
- Laidlaw BJ, Zhang N, Marshall HD, et al. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity 2014;41:633-45. [Crossref] [PubMed]
- Bernatchez E, Gold MJ, Langlois A, et al. Pulmonary CD103 expression regulates airway inflammation in asthma. Am J Physiol Lung Cell Mol Physiol 2015;308:L816-26. [Crossref] [PubMed]
- Lohmeyer J, Friedrich J, Grimminger F, et al. Expression of mucosa-related integrin alphaEbeta7 on alveolar T cells in interstitial lung diseases. Clin Exp Immunol 1999;116:340-6. [Crossref] [PubMed]
- Kolopp-Sarda MN, Kohler C, De March AK, et al. Discriminative immunophenotype of bronchoalveolar lavage CD4 lymphocytes in sarcoidosis. Lab Invest 2000;80:1065-9. [Crossref] [PubMed]
- Heron M, Slieker WA, Zanen P, et al. Evaluation of CD103 as a cellular marker for the diagnosis of pulmonary sarcoidosis. Clin Immunol 2008;126:338-44. [Crossref] [PubMed]
- Mota PC, Morais A, Palmares C, et al. Diagnostic value of CD103 expression in bronchoalveolar lymphocytes in sarcoidosisc. Respir Med 2012;106:1014-20. [Crossref] [PubMed]
- Braun RK, Foerster M, Grahmann PR, et al. Phenotypic and molecular characterization of CD103+CD4+ T cells in bronchoalveolar lavage from patients with interstitial lung diseases. Cytometry B Clin Cytom 2003;54:19-27. [Crossref] [PubMed]
- Heron M, Grutters JC, Van Moorsel CH, et al. Effect of variation in ITGAE on risk of sarcoidosis, CD103 expression, and chest radiography. Clin Immunol 2009;133:117-25. [Crossref] [PubMed]
- Bretagne L, Diatta ID, Faouzi M, et al. Diagnostic value of the CD103+CD4+/CD4+ ratio to differentiate sarcoidosis from other causes of lymphocytic alveolitis. Respiration 2016;91:486-96. [Crossref] [PubMed]
- Danila E, Jurgauskienė L, Norkūnienė J, et al. BAL fluid cells in newly diagnosed pulmonary sarcoidosis with different clinical activity. Ups J Med Sci 2009;114:26-31. [Crossref] [PubMed]


