
Autologous blood patch pleurodesis for prolonged postoperative air leaks
Introduction
A prolonged air leak (PAL) is the most frequent complication after pulmonary resection, with an incidence of 7.6% reported in the ACOSOG Z0030 trial (1). Although most air leaks will heal spontaneously, a PAL, defined as lasting longer than five days, leads to a longer hospital length of stay (LOS) (2,3). Although preoperative risk factors for PAL have been identified, the postoperative management varies between clinicians (4). In addition to chest tube (CT) management, modalities such as chemical pleurodesis, Heimlich valve/pneumostat, pleural tents, endobronchial valve (EBV) placement, and surgery have been employed to hasten the resolution of air leaks or allow patients to discharge to home earlier (5). While discharge to home with an indwelling tube may allow patients to go home earlier, few studies have demonstrated effective prevention of air leak.
Pleurodesis with autologous blood patch pleurodesis (ABPP) occurs through multiple mechanisms (6). Direct application of a clot allows for the fibrogenic activity of a patient’s own blood to create a seal by irritating the pleura and causing an inflammatory cascade to ultimately seal the air leak. When small spaces exist between the chest wall and pleura, it is also possible that the act of filling the space with clot can enhance sealing through direct opposition against the leaking pleura. This technique is also cost-effective, in that the product comes from the patient and is always readily available.
The objective of this study was to review a single tertiary center’s experience using ABPP to treat PAL after lung resection for cancer or indeterminate pulmonary nodules (IPNs). We hypothesized that ABPP can decrease hospital LOS, shorten time to CT removal, and reduce the development of infectious complications.
We present the following article in accordance with the STROBE reporting checklist (7) (available at http://dx.doi.org/10.21037/jtd-20-1761).
Methods
Pleurodesis
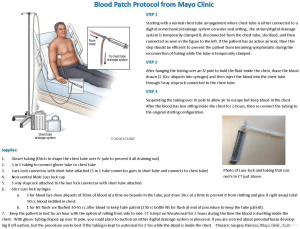
Per our institution’s protocol for ABPP administration, the patient is positioned with the unaffected side dependent or down, Figure 1. A laboratory technician then draws 90 cc of autologous blood into three equal 30 cc unheparinized syringes. The autologous blood sample is directly injected into the CT through a connector with stopcock followed by saline flush solution. Occasionally, the syringes are not completely full and thus the volume may vary to a minor degree. The CT then remains connected to waterseal off suction but with the tubing draped over an IV pole for at least 1–2 hours, turning to different positions every 15 minutes. No sedation or analgesia is required. There were no documented incidences of CT blockage with our protocol prior to implementation. If the patient is a Jehovah’s Witness, we modify the protocol and connect the blood tubing directly to the CT with a three-way stopcock which allows an unbroken tubing connection between the blood draw from the patient and the pleural space.
In this study, ABPP was performed during the immediate postoperative hospitalization period. Success of ABPP was defined as resolution of an air leak within 72 hours. Resolution was further classified as occurring within a 24-, 24–48-, or 48–72-hour time period as clinically assessed by the surgical team. Additional methods to resolve the air leak, such as deployment of EBVs, pneumoperitoneum, placement of additional chest tubes, or postoperative re-intervention were recorded. Patients discharged with a CT received post-discharge antibiotics per physician preference, as there was not a protocol for patients to routinely receive antibiotic therapy with an indwelling tube.
The decision about when to perform an ABPP was clinically determined by individual surgeon preference. ABPP utilized after postoperative day (POD) 5 when a patient failed conservative management with CT placement to wall suction (−20 mmHg) comprised the treatment group.
Data collection
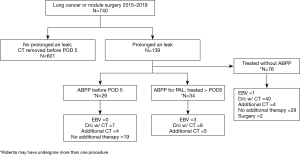
The study was conducted accordance with the Declaration of Helsinki (as revised in 2013) and approval obtained by the Mayo Institutional Review Board (IRB #16-005562). Individual consent for this retrospective analysis was waived. Using electronic medical health records, a retrospective review was conducted of patients undergoing surgery for lung cancer or IPN in a single tertiary care center between January 1, 2015 and April 30, 2019, Figure 2. In order to avoid selection bias, all patients meeting inclusion criteria from the thoracic surgery division were reviewed, and those who developed a postoperative air leak were identified. Baseline characteristics, operative data, and postoperative outcomes were collected. Surgery was classified as diagnostic or therapeutic and was grouped into open or minimally invasive (video-assisted or robotic) approaches. Patients presenting with infectious etiology prior to their lung surgery, such as a decortication or empyema, were excluded. Primary spontaneous and secondary pneumothorax related to underlying emphysema as well as interstitial lung disease patients were excluded in this study. Patients with a history of chronic obstructive pulmonary disease were included and forced expiratory volume/1 second (FEV1) was categorized similar to previous studies (3). If sealants were used at the time of surgery, these patients were excluded from the study as well.
As per the Society of thoracic Surgeons (STS) database definition, those patients with a PAL were defined as having an air leak persisting until at least POD 5 (8,9). Patients who did not undergo ABPP for a postoperative PAL comprised the control group. Descriptive data were collected for patients who received an ABPP at any point after surgery, but statistical analysis was performed only on those patients with an air leak beyond five days for the ABPP group. Those patients undergoing ABPP prior to POD 5 were excluded in the statistical analysis. The degree of pulmonary expansion was documented radiographically prior to ABPP, and then was re-reviewed by an expert thoracic-focused radiologist. Residual pleural space was measured in centimeters (cm) from the apex of the lung to the highest extent of chest wall apex on upright chest X-ray. The radiologist’s interpretation of the residual pleural space size was used to correlate the measurements into categories, (none/tiny ≤0.5 cm, 0.5–2 cm = small, 2–4 cm = moderate, and >4 cm = large). CT removal criteria included transition to waterseal for at least 4–6 hours, lack of an air leak with forced expiratory maneuvers, less than 300 cc of non-infected fluid output over a 24-hour period, and expansion of the lung on chest X-ray, as per protocol. Chest X-ray is routinely obtained 2–4 hours after CT removal; this was used to confirm documentation of date of CT removal and resolution of air leak. Readmission and reoperation data were collected for complications related to air leak in the postoperative period. During this same duration, we had a readmission quality improvement project where patients were contacted by telephone to determine if they were re-admitted to another outside facility. Complications managed at outside institutions were captured using electronic medical record review, telephone interviews, and documented follow-up visits.
Data analysis
Descriptive statistics were reported as number (percent) for discrete variables and as either mean (SD) or median (range) as appropriate for continuous variables. Since the decision of utilizing ABPP versus no ABPP for each patient was not randomized, we used inverse probability weighting (IPTW) to try to account for differences in baseline characteristics between treatment groups and prevent potential confounders. Using SAS version 9.4 software, a logistic regression model was utilized to obtain the predicted probabilities of treatment with ABPP. This model included the variables of age, sex, cancer status, surgery type (open vs. VATS), smoking status (non- vs. former vs. current), use of steroids, extent of resection (lobe vs. segment vs. other), location of lung resection (lower vs. middle vs. upper vs. multiple), FEV1 category (<60, 60–79, ≥80), and year of surgery, the model c-statistic was 0.88. Patients with incomplete data were excluded. The cumulative probability of hospital discharge, survival free of readmission, and cumulative probability of CT removal were estimated using the Kaplan Meier survival method. Univariate Cox models accounting for IPTW were used to assess the association of ABPP with each of the outcomes. An alpha-level of 0.05 was set for statistical significance.
Results
Patient selection
Over at least a four-year period, there were 110 (15%) patients who developed prolonged postoperative air leaks after lung resection for malignancy or IPN and met inclusion criteria. Of these, 34 patients underwent ABPP for PAL using 90cc of autologous blood. The remaining 76 patients did not undergo ABPP as dictated by surgeon preference and comprised the “no ABPP group” in this study (Figure 2). A total of 66 patients underwent ABPP but 29 of these patients had an initial ABPP before POD 5 due to physician preference and were thus excluded in the propensity-matched analysis. Additionally, 3 patients received ABPP and had resolution of an air leak prior to POD 5, not meeting the criteria for a PAL and were thus also excluded.
Effectiveness of ABPP
For a PAL, the first ABPP administration was successful in 22/34 patients (64%), and 21/22 (95%) of these patients had resolution of the air leak within the first 24 hours, Table 1. Of the remaining 12/34 patients, three were successfully treated with a second ABPP, four air leaks resolved with additional time (>48 hours) after initial ABPP, one had successful and one had unsuccessful resolution after EBV placement, and four patients were discharged with a pigtail catheter or Heimlich valve. Of the four patients discharged with a PAL, one patient required reoperation and one had EBV placement with resolution of air leak. Every patient in the ABPP group ultimately had successful CT removal within 30 days. Comparatively, in the no ABPP group (n=76), 40 patients were discharged with a Heimlich valve or pigtail catheter, one underwent EBV placement, six required surgery, four had an additional CT placed and 29 ultimately resolved with prolonged CT management with waterseal or suction. In this group 68/76 (89%) had removal of all chest tubes within 30 days. Table 1 additionally describes results for all ABPP treated patients, which includes those with ABPP administration prior to POD 5. In this group there was a 59% air leak resolution with the first ABPP compared with 64% when ABPP was used after POD 5.
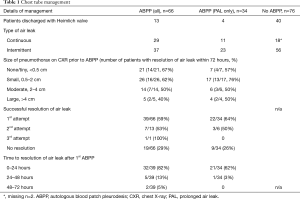
Full table
Residual pleural space size prior to administration of ABPP is described in Table 1. If a tiny or small space was present on chest X-ray prior to administration of ABPP, the success rate for air leak resolution within 72 hours was higher than if a moderate or large residual pleural space was present. This study was underpowered to delineate a benefit based on remaining pneumothorax measured as pleural space size.
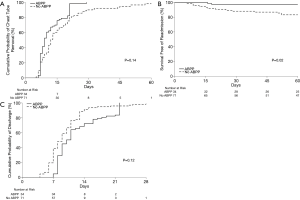
There was no significant difference in clinical variables between the no ABPP group and the group treated with ABPP in terms of age, gender, smoking history, preoperative FEV1, etiology necessitating lung resection, surgical approach, and type of resection (Table 2). It should be noted that over time, there was increase in surgeon preference to utilize ABPP in managing air leaks (Table 2). Although not significant, the ABPP group had a mean of 11 days before successful CT removal after surgery compared to a mean of 16 days for the no ABPP group (P=0.14, HR 1.52) (Figure 3A,B,C; Table 3).
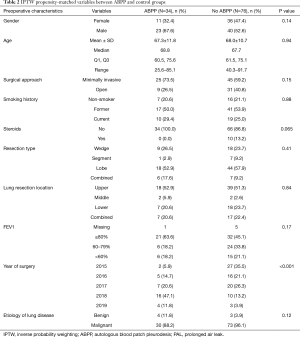
Full table

Full table
Patients with missing variables used in IPTW estimation of weights were not included in the analyses, which left 34 patients in the ABPP group and 71 patients in the no ABPP group. Analysis did not reveal a significant difference between the ABPP and no ABPP groups for in-hospital complications (P=0.18) and post-discharge complications (P=0.13), though, among ABPP treated patients, the risk of hospital readmission (P=0.02) was significantly lower. Empyema developed in 4 patients in the no ABPP group compared to 0 patients in the ABPP group (P=0.39, HR 0.24). Reoperation for air leak or empyema treatment was more often necessary in the no ABPP group (P=0.052, HR 0.1). The ABPP treatment group had a non-significant longer time to discharge after surgery (P=0.13, HR 0.63), with rates of discharge within 21 days of 83.7% and 95.9% in ABPP and no ABPP groups respectively (Table 4).

Full table
Follow-up data
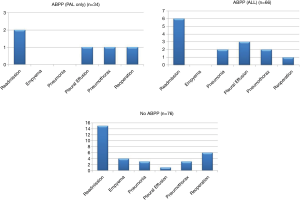
As seen in Figure 2, of the 740 patients undergoing surgery for a lung nodule or cancer, 66 patients were treated with ABPP. This study only included those patients who presented with a PAL and then were administered ABPP after the PAL was diagnosed. Three patients in our cohort had less than 30-day follow-up but did have a postoperative visit 6–14 days after discharge and did well without complications at the time of their last visit. In the control group, two patients were lost to follow-up, six patients were seen between 5–24 days after discharge, and 68 had at least 30 days’ follow-up. The numbers of total postoperative complications are illustrated in Figure 4 for each group; some patients may have had more than one postoperative complication. No empyema was found in the ABPP group, while four patients in the control group were diagnosed and treated for an empyema.
Discussion
This retrospective matched study of patients undergoing ABPP demonstrated fewer admissions and reoperations for PAL compared to those who did not undergo ABPP. The safety of this technique is also demonstrated by no increase in empyema-related complications.
In 1987, Robinson et al. were the first to report an efficacy of 85% (21/25 patients) using ABPP for spontaneous pneumothorax, followed by Dumire et al. extending the application to postoperative air leaks in 1992 (10,11). Contemporary data show a success rate of 75–93%, while empyema rates are reported around 9% (8,12-14). However, if no blood patch is performed, a PAL managed with a Heimlich valve has a 10–17% risk of associated empyema (15,16). An ongoing randomized trial is underway to determine if antibiotic therapy can reduce postoperative empyema in patients discharged to home with a PAL and CT (clinicaltrials.gov, NCT 03943511). Recent literature finds ABPP has a lower empyema rate reported in both retrospective trials and in a single randomized control trial (RCT) (17).
Reinersman et al. found an increase in dismissal with CT for PAL rising from 3.4% to 4.5% (P=0.03) over a 10-year period and discharging patients with a CT has an associated increased risk (17%) for development of empyema (16). Our study found a 4% incidence of empyema in patients who discharged with a CT compared to 0% who underwent ABPP treatment, suggesting an air leak itself may be a potential source to contaminate the pleural space.
Topical biodegradable sealants, such as ProGEL, have yet to demonstrate cost-effectiveness and studies have yet to demonstrate efficacy of EBV for PALs (18,19). Traditionally, chemical pleurodesis such as talc and doxycycline have been successful, but surgeons have been reluctant to use these agents due to side effects such as intense pain or acute respiratory distress syndrome (20). The lack of a standardized PAL pathway creates an environment where heterogeneous management takes place. ABPP is easily accessible and inexpensive, prevents the need for foreign material to be introduced into the chest, does not require sedation, and can be administered bedside with minimal discomfort (21). ABPP supplies and source are always readily available at any hospital, making it a more attractive option for pleurodesis. The time to perform this procedure is relatively quick, 10-20 minutes are required to set up and complete, with a 1–2-hour dependent CT dwell period.
PAL can prolong hospital stay and increase infectious complications. Brunelli et al. report their postoperative hospital course is prolonged from 8 to 16 days when a PAL is present, while others report a PAL was associated with a 5-day longer hospitalization (3,15). Our study did not find a significantly different LOS among patients in either ABPP versus no ABPP. However, there was a much higher incidence of outpatient management with a CT, 52% (40/76) in the no ABPP group compared to 18% (4/34) in the ABPP treated group. Within our comparison groups, LOS was confounded by providers waiting until POD 5 to administer an ABPP, which delayed discharge by another 1–2 days in order to observe for resolution of the air leak in contrast to the no ABPP patient group who discharged with a CT but continued to have an air leak on POD 5 while at home.
Compared to LOS, duration of CT may be a better indicator of air leak resolution. This study found administration of ABPP had a higher probability of earlier CT removal compared to the no ABPP group with a propensity-matched hazard ratio (HR) of 1.52 (CI: 0.866–2.676, P=0.14). Although this is not statistically significant, the findings are clinically relevant due to a significant HR and the fact that the study was underpowered to demonstrate a difference (powered to show a difference of only 5.8 days).
PAL has been associated with higher odds of readmission (OR =2, P=0.009) and empyema (OR =8.5, P<0.01) (3). Our study found a lower rate of 30-day readmission and reoperation in the ABPP group, indicating that ABPP effectively can seal a PAL to prevent subsequent complications of a PAL as identified by Attaar et al.
During conduct of the study there was no formal PAL protocol to guide management. Provider intervention with ABPP ranged from 2–13 days from surgery. In successful ABPP cases [64% (22/34) of patients with PAL], resolution occurred in almost all of these patients within 24 hours (21/22, 95%). Others report significant benefit (duration of air leak, CT removal, and LOS) with ABPP applied as early as POD 3 (20).
This study focused on assessment of ABPP on PAL after lung resection for benign or malignant lesions. In this series, none of the ABPP patients developed a postoperative empyema. In fact, our study showed that, without intervention for a PAL, there was a higher risk of empyema development. Therefore, even if ABPP is not effective in sealing all air leaks, the potential benefit is greater than the risk of empyema development. Importantly, PAL’s are routinely encountered by thoracic surgeons in various spectrums of practice where the generalizability of the ABPP methods and results can be particularly impactful.
Limitations and future direction
The authors recognize that findings are limited by the retrospective and underpowered nature of this study. Unfortunately, there is significant variability among thoracic surgeons on how to manage postoperative air leaks and when to intervene. This can lead to selection bias and differences in individual surgeon outcomes. Additionally, the implementation of ABPP in patients prior to identification of a PAL further skewed the results of this retrospective review, making the analysis difficult. Understanding these limitations, we found the prevalence of postoperative air leaks was problematic enough that an analysis of our outcomes was crucial to guide practice.
Based on the results of this study, our group aims to initiate a RCT to study the utility of ABPP after lung surgery. Objective measurements to quantify an air leak were not available in this study but, for a future trial, a digital chest drainage device will be used. Findings from a larger RCT will be essential to determine an algorithm and to standardize PAL management.
Conclusions
ABPP administration is safe compared to traditional PAL management. The ABPP group had no postoperative empyema complications and was less likely to need readmission or reoperation for PAL. Larger powered studies may demonstrate what appears to be earlier CT removal among patients treated with ABPP.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1761
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1761
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-20-1761
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1761). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted accordance with the Declaration of Helsinki (as revised in 2013) and approval obtained by the Mayo Institutional Review Board (IRB #16-005562). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 2006;81:1013-9. [Crossref] [PubMed]
- Okada S, Shimada J, Kato D, et al. Prolonged air leak following lobectomy can be predicted in lung cancer patients. Surg Today 2017;47:973-9. [Crossref] [PubMed]
- Attaar A, Winger DG, Luketich JD, et al. A clinical prediction model for prolonged air leak after pulmonary resection. J Thorac Cardiovasc Surg 2017;153:690-699.e2. [Crossref] [PubMed]
- Seder CW, Basu S, Ramsay T, et al. A Prolonged Air Leak Score for Lung Cancer Resection: An Analysis of The Society of Thoracic Surgeons General Thoracic Surgery Database. Ann Thorac Surg 2019;108:1478-83. [Crossref] [PubMed]
- Gillespie CT, Sterman DH, Cerfolio RJ, et al. Endobronchial valve treatment for prolonged air leaks of the lung: a case series. Ann Thorac Surg 2011;91:270-3. [Crossref] [PubMed]
- Manley K, Coonar A, Wells F, et al. Blood patch for persistent air leak: a review of the current literature. Curr Opin Pulm Med 2012;18:333-8. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. PLoS Med 2007;4:e296 [Crossref] [PubMed]
- Chambers A, Routledge T, Bille A, et al. Is blood pleurodesis effective for determining the cessation of persistent air leak? Interact Cardiovasc Thorac Surg 2010;11:468-72. [Crossref] [PubMed]
- The Society of Thoracic Surgeons. General Thoracic Surgery Database Data Collection. Available online: https://www.sts.org/registries-research-center/sts-national-database/general-thoracic-surgery-database/data-collection. Accessed 20th July 2020.
- Robinson CL. Autologous blood for pleurodesis in recurrent and chronic spontaneous pneumothorax. Canad J Surg 1987;30:428-9. [PubMed]
- Dumire R, Crabbe MM, Mappin FG, et al. Autologous “blood patch” pleurodesis for persistent pulmonary air leak. Chest 1992;101:64-6. [Crossref] [PubMed]
- Cagirici U, Sahin B, Cakan A, et al. Autologous blood patch pleurodesis in spontaneous pneumothorax with persistent air leak. Scand Cardiovasc J 1998;32:75-8. [Crossref] [PubMed]
- Cobanoglu U, Melek M, Edirne Y. Autologous blood pleurodesis: A good choice in patients with persistent air leak. Ann Thorac Med 2009;4:182-6. [Crossref] [PubMed]
- Droghetti A, Schiavini A, Muriana P, et al. Autologous blood patch in persistent air leaks after pulmonary resection. J Thorac Cardiovasc Surg 2006;132:556-9. [Crossref] [PubMed]
- Brunelli A, Xiume F, AlRefai M, et al. Air leaks after lobectomy increase the risk of empyema but not of cardiopulmonary complications: a case-matched analysis. Chest 2006;130:1150-6. [Crossref] [PubMed]
- Reinersman JM, Allen MS, Blackmon SH, et al. Analysis of Patients Discharged From the Hospital With a Chest Tube in Place. Ann Thorac Surg 2018;105:1038-43. [Crossref] [PubMed]
- Shackcloth MJ, Poullis M, Jackson M, et al. Intrapleural instillation of autologous blood in the treatment of prolonged air leak after lobectomy: a prospective randomized controlled trial. Ann Thorac Surg 2006;82:1052-6. [Crossref] [PubMed]
- Park BJ, Snider JM, Bates NR, et al. Prospective evaluation of biodegradable polymeric sealant for intraoperative air leaks. J Cardiothorac Surg 2016;11:168. [Crossref] [PubMed]
- Hance JM, Martin JT, Mullett TW. Endobronchial valves in the treatment of persistent air leaks. Ann Thorac Surg 2015;100:1780-6. [Crossref] [PubMed]
- French DG, Plourde M, Henteleff H, et al. Optimal management of postoperative parenchymal air leaks. J Thorac Dis 2018;10:S3789-98. [Crossref] [PubMed]
- Ibrahim IM, Elaziz MEA, El-Hag-Aly MA. Early Autologous Blood-Patch Pleurodesis versus Conservative Management for Treatment of Secondary Spontaneous Pneumothorax. Thorac Cardiovasc Surg 2019;67:222-6. [Crossref] [PubMed]


