Computed tomography guided microcoil localization for pulmonary small nodules and ground-glass opacity prior to thoracoscopic resection
Introduction
Video-assisted thoracoscopic surgery (VATS) has become the main surgical intervention tool for diagnosis and treatment of small pulmonary nodules and ground-glass opacity (GGO). However, intraoperative localization of small pulmonary lesions tends to be difficult (1). Preoperative localization is very helpful for guiding resection through VATS, which mainly adopts computed tomography (CT)-guided percutaneous lung puncture technology by placing a medium in the lung for localization of intraoperative lesions (2). The selection of medium is the key for localization efficacy. In 1994, Asamura first reported the use of a platinum microcoil, which is usually intended for embolization of selective vessel supply, for positioning small pulmonary nodules (3). Powell developed a better method by deploy the microcoil with the end coiled in the pleural space (4). We have performed preoperative microcoil localization since 2012 in our institution. And we adopted a way to deploy the microcoil somewhat different from Powell’s method. In this study, we introduce our method and evaluate the feasibility, safety and efficacy of preoperative microcoil localization for small pulmonary lesions in our single institution.
Methods
Study subjects
Retrospectively reviewed the data of patients with small pulmonary solid nodules and GGO who underwent microcoil localization prior to thoracoscopic surgery from March 2013 to November 2014 in Peking University People’s Hospital, Beijing, China. Preoperative localization using microcoil was conducted in lesions according to the following conditions: (I) solid nodules with a diameter ≤1 cm and distance to visceral pleura ≥0.5 cm; (II) GGO; (III) part-solid GGO, with a solid portion ≤1 cm and distance to the visceral pleura ≥1 cm. Of all the patients, patients who underwent preoperative localization by deploying the end of microcoil coiled outside lung parenchyma were included in this study. As standard of care, all patients signed informed consent form before preoperative localization. Our institutional review board approved the present retrospective study and waived the requirement for informed consent for collecting medical data from the related patients.
Microcoil localization
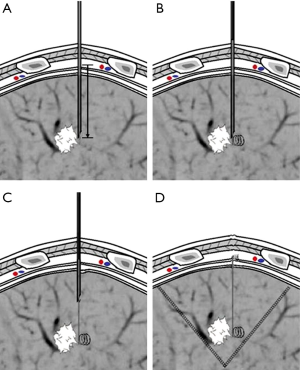
Patients underwent CT-guided percutaneous pneumocentesis for positioning within three days prior to surgery at our radiology department. Embolization Microcoil (Cook incoporated, Bloomington, IN 47404, USA) was selected as positioning markers, with a wire diameter of 0.18ʹʹ and a length of 7 cm. A percutaneous introducer kit (Argon Medical Devices Inc., Athens, TX75751, USA) with a 21 G puncture needle and a 45 cm ×0.18ʹʹ guide-wire) was used. Before puncturing, the desired length of the guide-wire was prepared with the whole length of the loading cannula connecting with the puncture needle. After local anesthesia with 2% lidocaine, the procedures are illustrated as follows: CT-guided percutaneous puncture was carried out using the puncture needle, during which the needle pathway avoided the lesions, and the tip was positioned in the normal lung parenchyma around the lesions. Successful puncture was confirmed by the CT scan and then the loading cannula of the microcoil was connected to the needle. Our method named “trailing” for deploy the microcoil was derived from Powell’s method (4). However In our method, it was intended that the proximal end of the microcoil be left on the parietal pleura: at first the distance from the needle tip to beyond the parietal pleura was measured, and marked on the guide-wire (Figure 1A). The guide-wire was then inserted into the needle and advanced to the marked location, pushing the distal part of the microcoil into the lung parenchyma (Figure 1B). The guide-wire was fixed in place and the needle was withdrawn slowly with the proximal part of the microcoil remained in the needle tubing (Figure 1C). When the needle was withdrawn to the desired length on the guide-wire, the needle and guide-wire were withdrawn simultaneously and the proximal part of the microcoil was deployed. Scanning was conducted to confirm the position of the microcoil, where technical success referred to the proximal end of the microcoil coiling beyond the parietal pleura while the distal part anchoring in the lung parenchyma (Figure 1D). The presence of pneumothorax and hemorrhage was also assessed. Later patients were sent back to the ward. Patients who did not intend to undergo surgery on the same day underwent chest radiographs on the morning of the day for surgery or at the time of occurrence of symptoms.
Thoracoscopic surgery
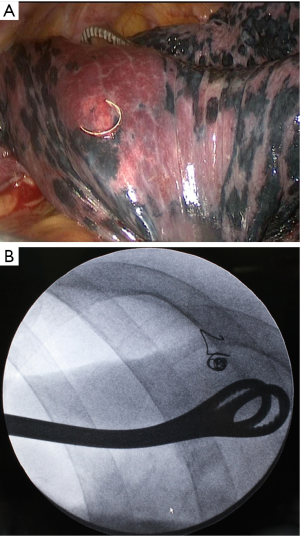
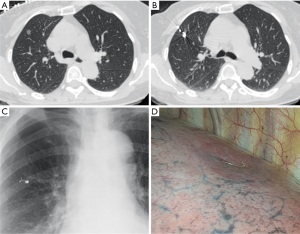
Conventional thoracoscopic surgery was adopted. The patients were on ventilator with a double lumen endotracheal tube under general anesthesia. Later patients were placed in the lateral position with ipsilateral one-lung ventilation and two to three ports were made. The observation port was located at the midaxillary line of the 7th or 8th rib, the main operating port was located at the anterior axillary line of the 4th or 5th rib with a length of 3-4 cm, while the auxiliary incision was at the infrascapular line of the 7th or 8th rib. First, visual examination was done, and then positioning was determined by looking for the proximal end of the microcoil beyond the visceral pleura. If the marker was not found, palpation was conducted on the microcoils or lesions bypassing the main operating port. For cases with successful location by visual inspection or palpation of the microcoil or pulmonary lesions, pulmonary wedge resection or pulmonary segmental resection using endoscopic staplers was performed. For cases with unsuccessful palpation, fluoroscopy was utilized to find the microcoil and then pulmonary wedge resection or pulmonary segmental resection was performed and the integrity of the coil was confirmed. The incised specimens were subjected to intraoperative consultation with frozen section. For pulmonary malignancy, frozen section diagnosis was made not only including the initial diagnosis of lung cancer, but also a distinguishment between in situ, minimally invasive, and invasive adenocarcinoma. Based on the results of frozen section diagnosis, the operation could be ended for patients with benign lesions or noninvasive lung cancer. Patients with invasive lung cancer underwent thoracoscopic lobectomy and lymph node dissection or sampling. Patients with suspected invasive lung cancer were treated by lobectomy or sublobar resection and lymph node sampling following their willing in the informed consent preoperatively. If lesions were found in a deep location and enough margin distances were not achievable for sublobar resection under fluoroscopic guidance (Figure 2), thoracoscopic lobectomy was conducted and lesions were identified by palpation of the microcoil after the specimens were isolated
Data collection and statistical analysis
Clinical data, imaging data, surgical data, and postoperative pathological information, which included age, sex, location of lesion, characteristics of lesion, diameter, complications of localization, surgical strategies, pathology, intraoperative positioning methods, etc. were collected. Success rate was compared using the Chi-square analysis, with the significance level set at 0.05. Excel 2010 and SPSS18.0 software were used for analyzing the data.
Results
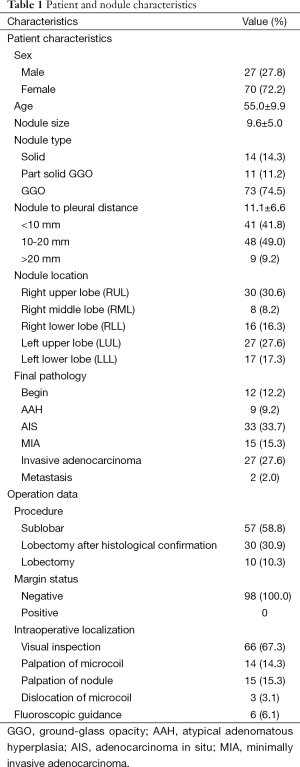
From March 2013 to November 2014, 1,219 patients with lung lesions underwent thoracoscopic surgery. A total of 97 patients (8.0%) were included in this study, of which 88 patients with solitary lesion, 8 patients with multiple nodules in same lobe and 1 patient with two separate nodules in ipsilateral different lobes. In total, 98 lesions were localized using the “trailing method” prior to thoracoscopic surgery. The demographic information is listed in Table 1. There were 14 solid nodules, 11 part-solid ground-glass nodules, and 73 pure ground-glass nodules, with a mean diameter of 9.6 mm (range, 4-26 mm). The mean distance from the lesions to the pleura surface was 11.1±6.6 mm.
Full table
CT-guided placements of microcoil were successful in all lesions. The technical success rate of deploying with the tail coiled beyond the parietal pleura was 85.7% (84/98) patients. Seventeen patients (17.3%) had mild complications detected by CT scan after the procedure of localization. Thirteen patients with asymptomatic pneumothorax, four patients with pulmonary hematoma. Of all the patients with mild complication, six (three asymptomatic pneumothorax, one symptomic pneumothorax, two pulmonary hematoma) underwent surgery on the next day of localization, and a chest X-ray was followed up before surgery. Only the patient with symptomic pneumothorax required further thoracentesis as lung collapse was greater than 50% and dyspnea was present.
All patients underwent thoracoscopic surgery; 42 patients (43.3%) underwent thoracoscopic surgery on the same day of localization, 49 patients (50%) on the next day, and 6 patients (6.1%) on the subsequent day. During the surgery, 66 lesions (67.3%) were localized through the proximal end of the microcoil beyond the visceral pleura by visual inspection, 29 lesions were localized by palpation of the microcoil, and 3 lesions had dislocation of the microcoil, of which two were localized by palpation, and one was localized by the hematoma at puncture site, resulting in a success rate of 96.9% for intraoperative microcoil localization. All small pulmonary nodules and GGO were successfully resected, with a surgical success rate of 100%. Surgical procedures were all completed under thoracoscopy. Fifty-seven patients underwent sublobar resection, 31 for wedge resection, and 26 for segmentectomy. Nineteen patients underwent lobectomy when frozen section proved primary invasive malignancy, 11 patients underwent lobectomy respecting their choices when frozen section diagnosis deferred in assessment of invasion, and 10 patients who underwent lobectomy directly due to multiple lesions or deep location. Postoperative pathological examination revealed 33 cases of adenocarcinoma in situ (AIS), 15 cases of minimally invasive adenocarcinoma (MIA), 27 cases of invasive adenocarcinoma, 9 cases of atypical adenomatous hyperplasia (AAH), 2 cases of metastasis, and 12 cases of benign nodule. No recurrence was found with a median follow-up of 9 months.
Discussion
Advantages of microcoil localization
The microcoil used in this study was a platinum wire for embolization of vessel supply in vascular intervention surgery. There were several reasons for selecting the microcoil for localization: (I) it is commonly used, easy to acquire and inexpensive compared with other special hook wires, spiral wires, radionuclides, etc.; (II) it is a clinically proven material that can safely be sustained in the human body for a long time; (III) after implantation, it coils in the lung with a certain degree of hardness and it is radiopaque, which enables positioning by visual inspection, palpation, and fluoroscopy during surgery; (IV) the placement operation is not complicated and has good repeatability. Microcoil localization can also make up for the deficiencies of other materials (5-10): (I) compared with the commonly used hook wire, the microcoil can be retained in the patients’ body and is not easily detached. Therefore, it is not necessary to perform surgery immediately after the localization; (II) compared with solvents such as iodine, complications caused by intravascular injection and solvent diffusion effects on localization need not be concerned. It is also suitable for patients with silicosis with deep pulmonary surface color and patients with chronic obstructive pulmonary disease; (III) compared with radionuclide, microcoil cannot be contamination through radiation, and does not require special equipment and personnel training. Therefore, it is believed that the microcoil is an ideal positioning material at present.
Principle of “trailing method”
In the first cases of microcoil localization in our institution, the entire microcoil was placed into the lung parenchyma, in which the microcoil was coiled as a helical configuration in the lung. Localization can be achieved using this method, but it is not intuitive and it requires intraoperative palpation or fluoroscopy. Powell et al. reported adjustment in the location of puncture needle tip using guidance through CT, and the microcoil was placed with the proximal end forming a compact helical configuration on the visceral pleural surface; hence localization could be achieved by visual inspection. The placement of the end of the microcoil in the chest wall was avoided in their study (4,11). In our method, it was intended that the proximal end of the microcoil be left on the parietal pleura. After the distal part of the microcoil was deployed and anchored in the lung parenchyma, the needle was withdrawn. The proximal end of the microcoil, which remained in the lumen of the needle, was stretched by the anchored part and presented a “comet tail” shape. Thus it was named as the “trailing method” (Figure 3). Since the guide-wire was introduced only beyond the parietal pleura, the proximal end of the microcoil was deployed beyond the parietal pleura as well. After performing one-lung ventilation and with lung collapse, the proximal end of the microcoil would hang on the chest wall or detach from the chest wall to the visceral pleural surface due to stretching by the pulmonary elastic recoil to facilitate intraoperative observation. Obviously the operation of “trailing method” is less complicated and can reduce the number of CT scans.
Success ratio of “trailing method”
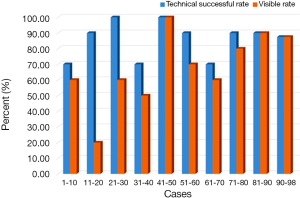
Since this novel “trailing method” was used for microcoil localization, the success rate reached 70% for the first ten placements, and similar results were obtained for every subsequent ten placements (P=0.231, Figure 4), which indicated that this method is easy to perform. However, the probability of observing the proximal end of the microcoil at the visceral pleura decreased after intraoperative one-lung ventilation. This phenomenon may be related to the following two reasons: first, the distal end of the microcoil detached from the lung and plunged to the pleura cavity, causing intraoperative positioning failure. There were three cases of dislocation in the present study; all were close to the visceral pleura. Seo et al. reported that the localization of the hook wire tip with sufficient depth from the pleural surface was crucial to the success of preoperative localization using hook wire (12). It was assumed that the cause of dislocation was to position the microcoil too close to the visceral pleura in this study. On the other hand, the main reason for failure to observe the proximal end of the microcoil was that the microcoil prematurely detached from the parietal pleura and retracted into the lung parenchyma due to its own elastic force. It was believed that the success rate can be improved by controlling the needling depth and length of the microcoil. In case of the former, it is not appropriate that the needle be close to the visceral pleura to avoid detaching from the lung and falling into the chest after delivery. However, too deep needling depth will increase risks of lung hematoma and bleeding (13). With regard to the length of the microcoil, a longer microcoil is preferred to ensure that the microcoil can coil into a larger ball and anchor in the lung to avoid causing dislocation, but it is also important to maintain sufficient length beyond the parietal pleura to prevent retraction of the microcoil in the lung. Referring to previous results, the depth of the needling tip should be between 1 and 2.5 cm (12,14) and the length of the microcoil should be longer than 6 cm when the “trailing method” was adopted. One aspect that needs to be emphasized is that the fixation of the puncture needle and guide-wire is the key for the “trailing method” since respiratory movement of the patients may cause changes in the needling depth in the process of delivery, resulting in placement failure.
Safety of microcoil localization
Microcoil localization was performed using CT-guided percutaneous puncture, which may present serious complications such as tension pneumothorax, bleeding, and even air embolism. Ichinose et al. performed percutaneous hook wire localization in 417 patients, and found that half of the patients presented with pneumothorax, of which 4.6% required pumping treatment, and the incidence of hemoptysis and hematoma was 10.3% while the incidence of air embolism was 0.24% (14). In this study, the complications of microcoil localization were also caused by puncture damage of lung tissues, of which pneumothorax usually occurred in lesions adjacent to the pleura or repeated puncture in multiple lesions, while hematoma was more common in lesions with deep location and longer traveling distance of needle, which was similar to previous results (13,14). Fewer complications were observed in our group compared with hook wire localization. This may be due to the smaller study size as well as early precaution and case selection using new technologies. It should be noted that though 52.9% (45/85) patients did not undergo surgery on the same day of positioning, more significant complications were not observed due to prolonged waiting time for surgery. It is believed that the structural characteristics of the microcoil might help in reducing the severity of complications. The thrombogenic coating of synthetic nylon fibers on the surface of the microcoil may promote blood coagulation of the surrounding lung tissues, block the needle pathway, and decrease the severity of pneumothorax and bleeding caused by the puncture needle damaging the lung tissues, which has been proven in animal experiments (15). However, the number of cases in the existing reports about microcoil localization was less (3,4,11,16) and there is lack of comparison with control groups. Further investigations are required to clarify its complications.
Indication of “trailing method”
In this study, the indication of preoperative localization was made based on the experiences of the thoracic surgeons in charge. During thoracoscopic exploration, 15/98 (15.3%) nodules was identified as palpable by the surgeons in charge, with 3/14 (21.4%) solid nodules, 1/11 (9.1%) part-solid ground-glass nodules, and 8 (12.0%) pure ground-glass nodules. Thus the indication of preoperative localization was considered reasonable though the detectability of solid nodules was better in the study. However, it should be reminded that the “trailing method” is not applicable for all lesions requiring localization. Generally speaking, lesions at deep location are usually removed by direct lobectomy, in which the microcoil will mark the lesion site to facilitate localization of the lesion in the resected specimen. In this circumstance, a visible tail of the microcoil is still helpful for quicker location in the resected specimen but it should be reminded hematoma is more common with greater needle insertion distance. If sublobar resection is to attempt for these deep lesions, a visible tail of the microcoil will facilitate defining both the location and the resection range of the nodules (Figure 2). The convenience of the “trailing method” is exceptional. However, the microcoil placed in the lung parenchyma has to be removed completely. The different routes for CT-guided percutaneous puncture and the endoscopic stapler placement may cause trouble for removing the microcoil placed by the “trailing method” in sublobar resection. In this study, lesions close to the scapula, armpit, and spine were found to be not suitable for the “trailing method”. In other words, lesions in these positions are proposed as a relative contraindication of the “trailing method” if sublobar resection is planned.
Conclusions
In summary, CT-guided microcoil localization by “trailing method” prior to thoracoscopic resection is a feasible, safe, and effective method for localization of pulmonary small nodules and GGO and warrants further investigation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Suzuki K, Nagai K, Yoshida J, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest 1999;115:563-8. [PubMed]
- Zaman M, Bilal H, Woo CY, et al. In patients undergoing video-assisted thoracoscopic surgery excision, what is the best way to locate a subcentimetre solitary pulmonary nodule in order to achieve successful excision? Interact Cardiovasc Thorac Surg 2012;15:266-72. [PubMed]
- Asamura H, Kondo H, Naruke T, et al. Computed tomography-guided coil injection and thoracoscopic pulmonary resection under roentgenographic fluoroscopy. Ann Thorac Surg 1994;58:1542-4. [PubMed]
- Powell TI, Jangra D, Clifton JC, et al. Peripheral lung nodules: fluoroscopically guided video-assisted thoracoscopic resection after computed tomography-guided localization using platinum microcoils. Ann Surg 2004;240:481-8; discussion 488-9. [PubMed]
- Choi BG, Kim HH, Kim BS, et al. Pulmonary nodules: CT-guided contrast material localization for thoracoscopic resection. Radiology 1998;208:399-401. [PubMed]
- Vandoni RE, Cuttat JF, Wicky S, et al. CT-guided methylene-blue labelling before thoracoscopic resection of pulmonary nodules. Eur J Cardiothorac Surg 1998;14:265-70. [PubMed]
- Dendo S, Kanazawa S, Ando A, et al. Preoperative localization of small pulmonary lesions with a short hook wire and suture system: experience with 168 procedures. Radiology 2002;225:511-8. [PubMed]
- Watanabe K, Nomori H, Ohtsuka T, et al. Usefulness and complications of computed tomography-guided lipiodol marking for fluoroscopy-assisted thoracoscopic resection of small pulmonary nodules: experience with 174 nodules. J Thorac Cardiovasc Surg 2006;132:320-4. [PubMed]
- Ciriaco P, Negri G, Puglisi A, et al. Video-assisted thoracoscopic surgery for pulmonary nodules: rationale for preoperative computed tomography-guided hookwire localization. Eur J Cardiothorac Surg 2004;25:429-33. [PubMed]
- Gonfiotti A, Davini F, Vaggelli L, et al. Thoracoscopic localization techniques for patients with solitary pulmonary nodule: hookwire versus radio-guided surgery. Eur J Cardiothorac Surg 2007;32:843-7. [PubMed]
- Mayo JR, Clifton JC, Powell TI, et al. Lung nodules: CT-guided placement of microcoils to direct video-assisted thoracoscopic surgical resection. Radiology 2009;250:576-85. [PubMed]
- Seo JM, Lee HY, Kim HK, et al. Factors determining successful computed tomography-guided localization of lung nodules. J Thorac Cardiovasc Surg 2012;143:809-14. [PubMed]
- Kim YD, Jeong YJ. Localization of pulmonary nodules with lipiodol prior to thoracoscopic surgery. Acta Radiol 2011;52:64-9. [PubMed]
- Ichinose J, Kohno T, Fujimori S, et al. Efficacy and complications of computed tomography-guided hook wire localization. Ann Thorac Surg 2013;96:1203-8. [PubMed]
- Gagliano RA Jr, Reinschmidt JP, Murray SP, et al. A novel method of transthoracic lung nodule localization. Current Surgery 1999;56:410-12.
- Lizza N, Eucher P, Haxhe JP, et al. Thoracoscopic resection of pulmonary nodules after computed tomographic-guided coil labeling. Ann Thorac Surg 2001;71:986-8. [PubMed]


