
Outcomes related to anticoagulation management for mechanical valve replacements
Introduction
The patient population requiring valve replacement is widely heterogeneous in age, comorbidities, and functional status. This adds complexity to decision-making for valve selection, particularly with the current availability of bioprosthetic, mechanical, and now transcatheter valve options. Recent literature has analyzed the effectiveness of transcatheter valves in low-risk and younger individuals, highlighting the possibility of a valve-in-valve approach to address future structural deterioration (1,2). Mechanical valves have historically been favored for younger patients due to long-term durability, however, their use necessitates lifelong anticoagulation with warfarin which imparts a risk of anticoagulation-related adverse events. Anticoagulation for mechanical valves requires diligent management to ensure patients are within the target international normalized ratio (INR) range for their specific valve type since rates of valve-related and anticoagulation-related adverse events have been correlated to the duration of time spent outside the therapeutic range (3). It is unknown whether slight deviations in INR may lead to adverse events that nullify the benefits associated with the durability of mechanical valves. A recent multicenter analysis demonstrated a survival benefit for mechanical valves in patients under 55 years undergoing mitral valve replacement (MVR) and under 70 years undergoing aortic valve replacement (AVR) (4). Thus, it is imperative to improve understanding of how variations in INR may impact survival and complications. The aim of this study was to examine anticoagulation-related events and their impact on longitudinal clinical outcomes among patients undergoing mechanical MVR or AVR. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-2562).
Methods
Study population
We examined adult patients (≥18 years) at our institution undergoing mechanical MVR or AVR between 2010–2018. Only mechanical valve types with target INR ranges of 2.0–3.0 for AVR and 2.5–3.5 for MVR were included. Valves such as the On-X valve were excluded due to infrequent use at our institution and lower suggested therapeutic ranges. Patients undergoing concomitant procedures were included. Those undergoing double mechanical valve replacement (AVR and MVR) were analyzed according to a target INR of 2.5–3.5. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board at the University of Pittsburgh approved this study (MOD18120143-003). Individual consent for this retrospective analysis was waived.
Approach to valve selection
Our institutional practice is to favor mechanical valve replacement in relatively healthy patients (life expectancy after valve surgery of at least 5 years) less than 70 years of age for mitral valves and less than 65 years of age for aortic valves. This practice stems from our belief in the durability advantage of mechanical valves particularly in the mitral position, our comfort level and favorable outcomes with reoperative valve surgery, and the unknown durability of transcatheter valve-in-valve procedures. We approach all valve selection discussions with a shared decision-making mentality and educate the patient on operative risks, redo operative risks versus transcatheter options in the future, and risks of anticoagulation.
Post-operative anticoagulation monitoring
Our clinical practice is to obtain daily INRs immediately post-operatively during the index hospitalization until the level is therapeutic, then biweekly following discharge. These values are managed by the surgeon and outpatient surgical team until a stable dose has been established. At that time, the INR management is transitioned to the patient’s primary care provider and INRs are checked monthly. Our institution ascribes to the following INR parameters outlined by the American Heart Association/American College of Cardiology (AHA/ACC): 2.0–3.0 (target 2.5) for AVR and 2.5–3.5 (target 3.0) for MVR (5). Additionally, we maintain all patients on aspirin 81 mg.
We reviewed all recorded INRs for each patient in the first post-operative year. These values were then stratified into five time periods relative to the operative date (0–1, 1–3, 4–6, 7–9, 10–12 months). We then determined the number of patients with at least one recorded INR, stratified by valve type (mitral or aortic). For each time period, we calculated the median and mean number of INRs recorded per patient (5). All patients with at least 5 INRs were then stratified into non-therapeutic and therapeutic INR groups. For each patient, a percentage of therapeutic INRs was determined using the following formula:
From these calculations, the population median of these patient-specific percentage of therapeutic INRs was obtained [median 42.86%, interquartile range (IQR): 30.77–53.95%]. Those patients whose individual therapeutic INR percentage fell within the population median and IQR (n=329, 50.85%) were categorized as therapeutic while those whose INR fell above or below the population median’s IQR (n=318, 49.15%) were categorized as non-therapeutic.
Statistical analysis
Baseline characteristics are presented as frequency (percentage) for categorical variables and mean [standard deviation (SD)] or median (IQR) for continuous variables based on normality. For categorical variables, Pearson’s Chi-square test or Fisher’s exact test was utilized for categorical comparisons whereas for continuous variables either Student’s t-test or Mann Whitney U test were employed. Kaplan-Meier analysis was utilized to model overall survival. Risk-adjusted long-term survival stratified by valve type and INR therapeutic categorization (non-therapeutic or therapeutic) was obtained from Cox regression analysis utilizing the following variables: age, sex, race, body mass index, body surface area, comorbidities, family history of coronary artery disease (CAD), New York Heart Association (NYHA) classification, cardiac presentation, operative status, serum albumin, ejection fraction, antiplatelet or anticoagulation use, endocarditis, and first operation or reoperation. Freedom from heart failure, stroke, major bleeding, arterial thromboembolism, and readmission for intravenous heparin were obtained from Cox regression models with the aforementioned input variables. The associated freedom from event curves were also plotted.
Results
Baseline characteristics
A total of 651 patients underwent mechanical valve replacement with 166 (25.5%) undergoing MVR and 485 (74.5%) undergoing AVR (Table 1). All prosthetic valves utilized in either the mitral or aortic position were St. Jude mechanical valves (either Masters or Regent series). MVR patients were significantly older (62 vs. 59 years, P=0.003), were more likely to be female (62.7% vs. 33%, P<0.001), and had a higher NYHA classification (P<0.001). There were no significant differences in the proportion of patients discharged on antiplatelet (P=0.29) or anticoagulation (P=0.32) medications. Among AVR patients, 81.9% had aortic stenosis (Table S1). Among patients undergoing MVR, 79.6% demonstrated either moderate or severe mitral regurgitation.
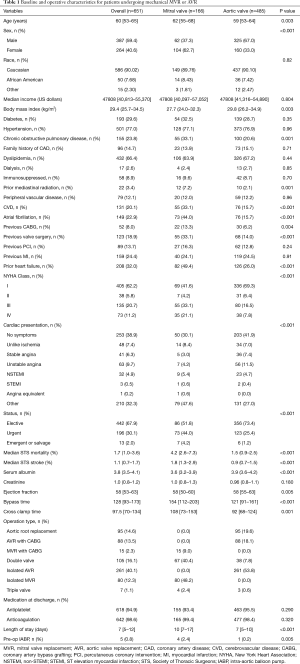
Full table
Anticoagulation monitoring
Of the 651 patients, 647 (99.4%) had at least 5 recorded INRs and were included in the therapeutic level analysis. A median of 27 (IQR: 14–42) INRs were drawn per patient in the first post-operative year with a median of 42.85% (IQR: 30.77–53.95%) of INRs falling within the reference range. The majority of non-therapeutic INRs were sub-therapeutic (34.51%; n=6,864). The percentage of patients with at least one recorded INR decreased from 100% in the first month to 60.26% and 53.05% by 10–12 months among MVR and AVR patients, respectively (Table 2). Depiction of the number of INRs below, within, or above the therapeutic range is shown for both MVR (Figure 1A) and AVR (Figure 1B). Within the first post-operative year, 31.08–43.00% of INRs in MVR patients fell within the therapeutic range for the 3-month intervals whereas for AVR this number ranged from 39.45–53.77% (Figure 1). The proportion of sub-therapeutic INRs ranged from 38.85–44.40% for MVR as compared to 21.82–38.62% for AVR.
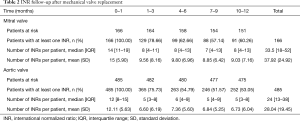
Full table

Post-operative outcomes
By univariate analysis, there were no significant differences in operative mortality, post-operative sepsis, reoperation, or stroke between MVR and AVR (Table 3). MVR patients were more likely to require blood products (49.4% vs. 24.3%, P<0.001) and prolonged mechanical ventilation (15.7% vs. 7%, P=0.001).
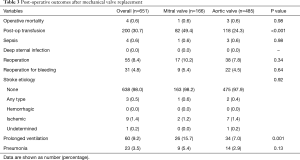
Full table
Survival and freedom from event analysis
Median follow-up was 2.72 years (IQR: 1.82–4.52). Overall survival was 95.74% at 1-year and 88.49% at 5-year (Figure 2A). Adjusted survival at 1- and 5-year was 97.43% and 91.79% for MVR compared to 98.29% and 94.48% for AVR (Figure 2B). Although MVR patients had higher hazards for longer-term mortality [hazard ratio (HR): 1.51, 95% confidence interval (CI): 0.77–2.94, P=0.23], this did not achieve statistical significance. Risk-adjusted survival based on patient classification as non-therapeutic or therapeutic anticoagulation was associated with a hazard of 1.12 (95% CI: 0.6–2.09, P=0.73) (Figure 3). Risk-adjusted survival at 1-year was 93.18% and 98.85% in the non-therapeutic and therapeutic groups, respectively, compared to 93.39% and 95.93% at 5-year. One-year event rates are shown (Table S2). Additionally, all-cause mortality and readmission rates are shown for the non-therapeutic and therapeutic groups, stratified by valve position (Table S3). In the event analysis, only heart failure readmission was associated with a greater hazard in the non-therapeutic group (HR: 1.21, 95% CI: 1.13–1.29, P<0.001) (Figure 4A). Adjusted HRs for stroke (HR: 1.83, 95% CI: 0.90–3.73, P=0.09, Figure 4B) and readmission for intravenous heparin (HR: 1.44, 95% CI: 0.92–2.25, P=0.11, Figure S1) were not significantly different. Freedom from bleeding (Figure S2A) and non-stroke arterial thromboembolism (Figure S2B) curves are also shown.
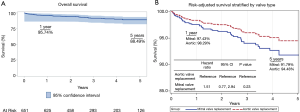


Conclusions
In this retrospective single-center analysis of patients undergoing mechanical valve replacement, we demonstrate comparable risk-adjusted survival and clinical outcomes when stratifying patients based on degree of therapeutic INRs in the first post-operative year. We found that less than half of INRs fell within the therapeutic range, with AVR patients more commonly achieving therapeutic levels. INRs outside of the normal range were more commonly sub-therapeutic, particularly in the MVR cohort. When stratified into therapeutic and non-therapeutic groups, the rates of thromboembolic events and bleeding at 1-year were comparable. Thus, despite variability in INRs, slight deviation outside the recommended therapeutic range, which is likely transient in nature, does not appear to contribute to worse outcomes.
Within the first post-operative year, only about half of INRs in AVR patients fell within the target range and these percentages were lower for MVR patients. This highlights the difficulty in attaining recommended INR targets, particularly among MVR patients (6). Time in the therapeutic range has been utilized to assess the relationship between anticoagulation and adverse outcomes, however, other studies have demonstrated that INR variability, as measured by the SD of the INR, may be a better predictor of mortality and complication rates (3,7,8). These analytical approaches underscore the accepted difficulty in maintaining target INRs. INR self-management, including self-monitoring and/or self-dosing, has demonstrated safety and feasibility, though a meta-analysis of patients managed with coumadin for any indication did not demonstrate a reduction in mortality (9-12). Our findings of a relatively consistent number of INR levels throughout the first post-operative year reflect provider attentiveness to the INR variability. It is possible that the convenience associated with INR self-monitoring, with dosing instructions provided by practitioners, would improve target INR percentages; this is a strategy we are currently trialing for selected patients.
We did not find an association between 1-year rates of adverse events when comparing patients with a greater proportion of therapeutic INRs in relation to those with more INR variability outside the therapeutic range. The exception to this was heart failure readmission, which was associated with an elevated hazard for those in the non-therapeutic group. These results are concordant with published work which has demonstrated a low risk of thromboembolic complications among patients with previously stable INR values (13). Multiple studies have explored the safety of targeting a lower INR range for mechanical valve patients and have demonstrated similar rates of thromboembolic events with an INR target of 1.5–2.5 for AVR and 2–2.5 for MVR (14,15). We found that non-therapeutic INR levels were more often sub-therapeutic and did not demonstrate an association between patients with more non-therapeutic levels and rates of thromboembolism. These comparable outcomes may be partially attributable to concomitant use of aspirin in our patients, which has been associated with a lower rate of thromboembolism (16). Nevertheless, studies reporting increased risk of thromboembolism associated with time outside of the current recommended INR ranges have tampered enthusiasm for universal adoption of these lower targets (3,8). Despite the conflicting nature of the available evidence, our findings of comparable thromboembolic events among those with therapeutic and non-therapeutic INRs suggest it may be reasonable for future, randomized studies to evaluate more flexible INR parameters.
In addition to comparable rates of adverse events, we found no association between overall survival and classification as therapeutic or non-therapeutic. Others have reported an association between deviance from target INR levels and death, though confounding between comorbidities and ability to reach a therapeutic INR may bias these results (17). In the LOWERING-IT mechanical AVR trial which randomized patients to a target INR of 1.5–2.5 (n=197) versus the standard 2.0–3.0 (n=199), after a median follow-up of 5.6 years there were only two deaths (1 thrombotic event, 1 hemorrhagic cerebral event), both of which were in the standard INR group (14). Nevertheless, current practice guidelines do not provide recommendations on mechanical or bioprosthetic valve choices in patients between the ages of 50–70 years, which leads to complex decision-making that requires the surgeon to weigh the risk of anticoagulation-related events with valve durability (5).
Survival after mechanical as compared to bioprosthetic valves has recently been evaluated by Goldstone et al. who found a mortality benefit for mechanical AVR up to 70 years and for mechanical MVR up to 55 years when compared to biologic prostheses (4). As expected, the authors found higher rates of bleeding and stroke among patients with a mechanical valve and higher rates of reoperation, especially in younger patients, with a bioprosthetic valve (4). Thus, for select age groups, mechanical valve durability outweighs the risks of anticoagulation-related adverse events, suggesting that attempts to further minimize these occurrences, potentially through novel anticoagulation drugs or improved consistency in achieving therapeutic INR levels, may further broaden the survival benefit of mechanical valves. The mortality impacts of newer transcatheter aortic bioprosthetic valves, when compared to mechanical valves in low-risk populations under 70 years, have yet to be evaluated. Improved understanding of the long-term outcomes in younger patients undergoing bioprosthetic valve implantation with subsequent valve-in-valve replacement, as compared to initial mechanical valve implantation, will help to elucidate the relative risks and benefits of either approach.
This study has several limitations. As patients were identified retrospectively, we were unable to account for decision-making leading to the choice for mechanical valve replacement thus this population likely represents a selected cohort who were determined preoperatively to tolerate long-term anticoagulation. A randomized trial would reduce potential biases in valve selection. We also do not have data to explore long-term hemodynamic performance of the valve in relation to anticoagulation targets nor do we have data on the specific cause of death. Additionally, we acknowledge that after INR levels have stabilized, many patients transition anticoagulation monitoring to their primary care providers who may be outside our hospital system. Obtaining INR values at all time points for some of these patients was therefore challenging, and in addition there was no standardization in time intervals for obtaining INRs. We felt that it was pertinent to report 5-year outcomes although we acknowledge that we only analyzed INR variability in the first post-operative year. Although there is a possibility of readmission or clinical events that occurred outside our hospital network and therefore were not captured, we have performed internal quality control analyses and have found that over 90% of such events are captured in our data management system, likely related to our 40-hospital regional network and the fact that most of our patients are covered under our system’s health insurance plan. Furthermore, we could not adjust for all factors that may have contributed to INR management, such as holding anticoagulation for procedures. Finally, granular details of the time sequence of non-therapeutic INRs and clinical events was not analyzed. In this respect, it is unclear if a non-therapeutic INR led to stroke, for example, or if the patient developed a stroke while therapeutic and their anticoagulation was held.
We report our experience with mechanical MVR and AVR anticoagulation in 651 patients and demonstrate no difference in longitudinal survival or risk of stroke, major bleeding, thromboembolism, or readmission for intravenous heparin based on the degree of therapeutic INRs in the first post-operative year. These data serve to alleviate some concern about the clinical impact of INR fluctuation, which is common among patients with mechanical valves. Future research endeavors targeting methods to ensure more consistent INRs and investigating the potential to expand the currently recommended INR therapeutic ranges are warranted.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-2562
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-2562
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-2562). TGG reports non-financial support from Abbott, Inc., outside the submitted work. AK reports personal fees from Medtronic Inc., outside the submitted work and he serves as an unpaid editorial board member of Journal of Thoracic Disease from Oct 2019 to September 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board at the University of Pittsburgh approved this study (MOD18120143-003). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Webb JG, Mack MJ, White JM, et al. Transcatheter aortic valve implantation within degenerated aortic surgical bioprostheses: PARTNER 2 valve-in-valve registry. J Am Coll Cardiol 2017;69:2253-62. [Crossref] [PubMed]
- Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019;380:1695-705. [Crossref] [PubMed]
- Havers-Borgersen E, Butt JH, Vinding NE, et al. Time in therapeutic range and risk of thromboembolism and bleeding in patients with a mechanical heart valve prosthesis. J Thorac Cardiovasc Surg 2019; Epub ahead of print. [Crossref] [PubMed]
- Goldstone AB, Chiu P, Baiocchi M, et al. Mechanical or biologic prostheses for aortic-valve and mitral-valve replacement. N Engl J Med 2017;377:1847-57. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;70:252-89. [Crossref] [PubMed]
- Le Tourneau T, Lim V, Inamo J, et al. Achieved anticoagulation vs prosthesis selection for mitral mechanical valve replacement: a population-based outcome study. Chest 2009;136:1503-13. [Crossref] [PubMed]
- Lind M, Fahlén M, Kosiborod M, et al. Variability of INR and its relationship with mortality, stroke, bleeding and hospitalisations in patients with atrial fibrillation. Thromb Res 2012;129:32-5. [Crossref] [PubMed]
- Labaf A, Själander A, Stagmo M, et al. INR variability and outcomes in patients with mechanical heart valve prosthesis. Thromb Res 2015;136:1211-5. [Crossref] [PubMed]
- Heneghan C, Ward A, Perera R, et al. Self-monitoring of oral anticoagulation: systematic review and meta-analysis of individual patient data. Lancet 2012;379:322-34. Erratum in: Lancet 2012;379:1102. [Crossref] [PubMed]
- Koertke H, Zittermann A, Wagner O, et al. Efficacy and safety of very low-dose self-management of oral anticoagulation in patients with mechanical heart valve replacement. Ann Thorac Surg 2010;90:1487-93. [Crossref] [PubMed]
- Koertke H, Minami K, Boethig D, et al. INR self-management permits lower anticoagulation levels after mechanical heart valve replacement. Circulation 2003;108:II75-8. [Crossref] [PubMed]
- Soliman Hamad MA, van Eekelen E, van Agt T, et al. Self-management program improves anticoagulation control and quality of life: a prospective randomized study. Eur J Cardiothorac Surg 2009;35:265-9. [Crossref] [PubMed]
- Dentali F, Riva N, Malato A, et al. Incidence of thromboembolic complications in patients with mechanical heart valves with a subtherapeutic international normalized ratio. J Thorac Cardiovasc Surg 2009;137:91-3. [Crossref] [PubMed]
- Torella M, Torella D, Chiodini P, et al. LOWERing the INtensity of oral anticoaGulant Therapy in patients with bileaflet mechanical aortic valve replacement: results from the "LOWERING-IT" Trial. Am Heart J 2010;160:171-8. [Crossref] [PubMed]
- Bal U, Aydinalp A, Yilmaz K, et al. The effects of a low international normalized ratio on thromboembolic and bleeding complications in patients with mechanical mitral valve replacement. J Cardiothorac Surg 2014;9:79. [Crossref] [PubMed]
- Dong MF, Ma ZS, Ma SJ, et al. Anticoagulation therapy with combined low dose aspirin and warfarin following mechanical heart valve replacement. Thromb Res 2011;128:e91-4. [Crossref] [PubMed]
- Grzymala-Lubanski B, Labaf A, Englund E, et al. Mechanical heart valve prosthesis and warfarin - treatment quality and prognosis. Thromb Res 2014;133:795-8. [Crossref] [PubMed]


