A systematic review and meta-analysis of video-assisted thoracoscopic surgery treating spontaneous pneumothorax
Introduction
Spontaneous pneumothorax is a common emergency presentation in the respiratory department. It is mainly caused by a sudden increase of pressure in the lungs caused by severe cough and breath-holding, and the rupture of the parenchyma of the lungs and pulmonary vesicles. Pressure rises after gas enters the pleural cavity, leading to respiratory and circulatory dysfunction (1). Spontaneous pneumothorax is classified into primary pneumothorax and secondary pneumothorax. Primary pneumothorax is more common in young patients, whereby under the condition of no exogenous factors affecting the lung parenchyma, patients’ pulmonary bullae spontaneously rupture (2). Secondary pneumothorax is more common in middle-aged and elderly patients, wherein generally, based on the lung parenchymal disease, bullae are damaged. Spontaneous pneumothorax is mainly primary pneumothorax, with a male to female ratio of about 6:1. It is more common in young males, and especially among those who smoke (3).
There are various treatment methods for spontaneous pneumothorax, including closed thoracic drainage, pleural fixation, thoracoscopic surgery, and traditional open thoracotomy (4). In the past, closed thoracic drainage and other treatments have been used to improve the patient’s condition, but they do not completely address the root of the disease; thus relapse can easily occur. Schnell et al. [2019] (5) pointed out that the use of closed thoracic drainage to treat pneumothorax was related to a recurrence rate of up to 30%. Therefore, thoracic surgery is the fundamental way to treat spontaneous pneumothorax. MacDuff et al. [2010] (6) used traditional thoracotomy to treat spontaneous pneumothorax, and in doing so achieved better therapeutic effects. Due to the large incision of thoracotomy, it is necessary to cut the muscles around the chest wall and use a stretcher to support it. Generally, the chest pain index after the operation is high and is long-lasting (7). Elderly patients give up treatment opportunities due to unbearable pain, while young patients are worried that the scars left after surgery will affect their appearance and refuse open thoracic surgery (8).
In the early 1990s, Schramel et al. [1996] (9) first used video-assisted thoracoscopic surgery (VATS) to treat spontaneous pneumothorax, and the therapeutic effect was very satisfactory. VATS is a new minimally invasive surgery for thoracic surgery. With the continuous improvement of medical equipment conditions, the treatment range of VATS has also expanded. With the continuous improvement of medical equipment, the treatment range of VATS has also expanded (10). With the advantages of less trauma, fewer complications, and quick recovery, VATS has gradually replaced traditional open thoracic surgery and is now widely used in clinical practice (11).
In recent years, related studies have reported that only 4.5% of patients had complications when using VATS to treat spontaneous pneumothorax (12). However, there is still a lack of randomized controlled trials (RCTs) to compare the clinical efficacy of VATS and non-VATS for spontaneous pneumothorax. To further explore the efficacy of VATS for spontaneous pneumothorax, we performed a meta-analysis to evaluate the published research since the establishment of the database. We hypothesized the effect of VATS in the treatment of spontaneous pneumothorax is more obvious than that of other treatment methods, and aimed to provide a scientific theoretical basis for the promotion of VATS.
We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/jtd-21-652).
Methods
Literature searching
We searched the databases PubMed, Medline, Cochrane Library, China National Knowledge Network (CNKI), Wanfang, VIP, and Google Academic. The retrieval time was from the establishment of each database to 20 December 2020. Composite logic retrieval and Boolean logic retrieval were used to select relevant literature. Chinese databases were searched by the combination of “Video-assisted thoracoscopic surgery”, “spontaneous pneumothorax”, and “thoracotomy”, while the English databases were searched for the terms “Video-assisted thoracoscopic surgery”, “spontaneous pneumothorax”, and “thoracotomy”. The quality of the literature was evaluated using the software RevMan 5.3 (Copenhagen: The Nordic Cochrane Center, the Cochrane Collaboration, 2014.
Each database was subjected to a joint search strategy of free words and subject words. After repeated literature searches, search engines were used to locate the articles. Experts and researchers in the field were contacted to maintain currency with the latest research progress.
Inclusion and exclusion criteria of literatures
The inclusion criteria were as follows: (I) literature exploring VATS for spontaneous pneumothorax; (II) RCTs; (III) pathological control analysis, with an index comparison that was reliable within the 95% confidence interval (CI); (IV) language of the literature was in both Chinese and English; (V) the diagnosis of spontaneous pneumothorax met the standards of the World Health Organization (WHO).
The exclusion criteria were as follows: (I) studies that were not relevant to this research; (II) literatures published in duplicate; (III) literature review, summary, or case report; (IV) literatures for which complete data could not be obtained by contacting the original author; (V) literatures published in languages other than Chinese and English.
The title, abstract, and full text of the articles were screened by two senior experts independently, and three preliminary experiments were confirmed before screening. If there were inconsistencies in opinion among the experts, a consensus conclusion was achieved through discussion, or a third expert was invited to arbitrate.
Literature screening method
Literature screening was mainly divided into three steps. First, the titles and abstracts of the research article were read, and literature that was irrelevant to this study was excluded. Then, the full text was read, data in the research results were analyzed, and any literature that did not meet the inclusion requirements was excluded. Finally, the quality evaluation was performed, and two experts independently extracted the data with a unified Excel table. It was crucial that three preliminary experiments had been performed before data extraction. If there were inconsistencies among experts, a consensus was sought through discussion, or a third expert was invited to arbitrate.
Data extraction
The data extracted from the included research included: (I) the title of the research paper; (II) the first author and publication year; (III) the name of the publication; (IV) time of publication; (V) general information of the research cohort such as the average age, gender, and surgery method; (VI) the grouping method of the experimental and control groups and the statistical method; (VII) the source, sample size, and outcome indicators of the cases.
Outcome indicators
The outcome indicators included operation time (min), length of hospital stay (d), complication rate (case), intraoperative bleeding volume (mL), recurrence rate (case), and chest tube removal time (d).
Literature quality assessment
The risk of bias was assessed by two experts at the same time, and if there was disagreement, the outcome was determined through discussion, or a third expert was asked to arbitrate. The quality evaluation of this study referred to the Oxford system score (JADAD score) in the Cochrane System Review Manual: whether an RCT was performed; whether the random method was adequately described; whether the blinding method was used appropriately; whether the baseline of the literature was similar and consistent; whether the reason for participants’ withdrawal was clearly described. A score of 2 or less was considered low-quality research, a score of 3–5 was medium-quality research, and a score of 6 or more was considered high-quality research.
Bias risk assessment
The risk of bias was assessed by two experts at the same time, and if the two disagreed, the outcome was determined through discussion, or a third expert was asked to arbitrate. In this work, the Cochrane Collaboration was used as a tool for “bias risk assessment” of RCTs. The evaluation criteria included generation of random sequence, blind method, hiding of allocation scheme, integrity of data results, and research results. The above five aspects were respectively judged as “high risk bias”, “low risk bias”, and “unclear”.
Statistical methods
The statistical software Stata SE release 12.0 (Stata Corp., College Station, TX, USA) was employed for statistical analysis. Odds ratio (OR) was adopted to evaluate the recurrence rate. Mean difference (MD) was adopted to evaluate operation time, length of hospital stays, intraoperative bleeding volume, and chest tube removal time. Rev Man 5.3’s risk of bias assessment chart was utilized to assess the bias risk of included literatures. Each effect was expressed using a 95% CI. When the heterogeneity test showed that P>0.1 and I2<50%, the fixed effects model was used for meta-analysis. When the heterogeneity test showed that P<0.1 and I2>50%, the random effects model was used for meta-analysis.
Results
Search results and basic information of included literatures
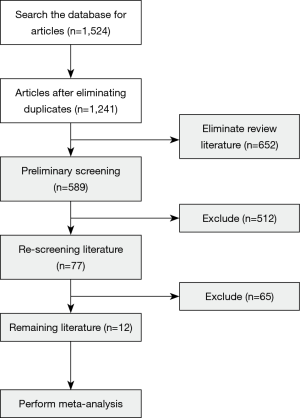
In total, 1,524 literatures were screened; among which, 1,447 were eliminated after the abstracts and titles had been read, and 65 were eliminated after the full text of the article was read. Finally, 12 literatures were included in the meta-analysis. Literatures were excluded for the following reasons: repeated research subjects (532 articles); literature type not case-controlled analysis (254 articles), the research object not VATS for spontaneous pneumothorax (481 articles), and the study-related information could not be extracted (263 articles) (Figure 1).
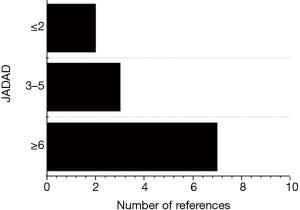
Figure 2 shows the quality classification results. There were 7 articles with a score of 6 or more, 3 with a score of 3–5, and 2 with a score of 2 or less.
A total of 12 articles met the inclusion criteria, including 8 retrospective analyses and 4 RCTs. A total of 744 participants were included. The experimental group was set as VATS for spontaneous pneumothorax, and non-VATS for spontaneous pneumothorax was set as the control group. Among the 12 articles, all were small sample studies, with sample sizes of 43–91 cases. The gender, age, number of cases, intervention measures, and outcome indicators of all articles were statistically analyzed. The general information of the research participants is shown in Table 1.
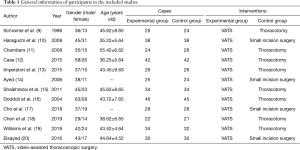
Full table
Results of literature risk bias evaluation
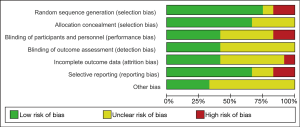
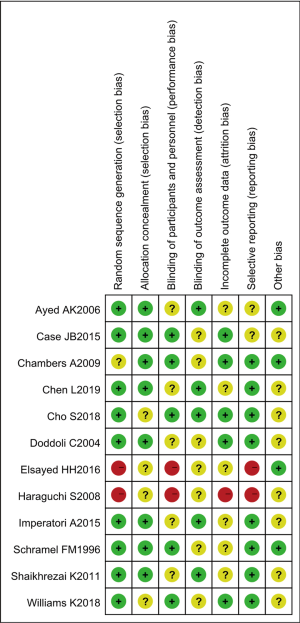
The results of multiple risk bias evaluations of literatures drawn by Rev Man 5.3 are shown in Figures 3 and 4. Among the 12 RCTs included in this work, 3 (13-15) described the correct random allocation method; 2 (16,17) described the correct random allocation method and described the concealment of the allocation plan in detail; 1 (18) was evaluated by blind method, and the other articles did not use a blind method. However, the measurement indicators in this work were laboratory indicators determined by computer, so it could be considered that all articles were blinded correctly.
Contrast of operation time
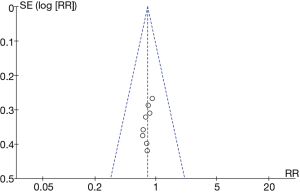
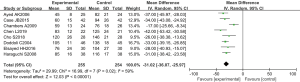
The operation time of RCTs was analyzed in eight studies. There were 509 cases in total, 255 cases in the experimental group, and 254 cases in the control group. The overall heterogeneity test showed that Tau2 =29.99, Chi2 =16.99, degrees of freedom (df) =7, and I2=59%>50%. Therefore, the random effects model was adopted for meta-analysis. The horizontal line of the 95% CI of all studies was to the left of the invalid vertical line. Meta-analysis showed that the operation time of the experimental group was greatly lower than that of the control group. The MD was −31.02, 95% CI: (−36.07 to −25.97), with statistically considerable differences (Z=12.03, P<0.01) (Figure 5).
A funnel plot of operative time was created using Rev Man 5.3 (Figure 6). It is obvious that the circle and the midline of some studies are basically symmetrical, suggesting that the research accuracy was high and there was no bias in publication.
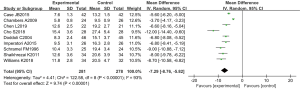
Contrast of length of hospital stay
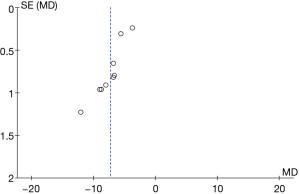
The length of hospital stay of RCTs was analyzed in 9 studies. There were 559 cases in total, 281 cases in the experimental group, and 278 cases in the control group. The overall heterogeneity test showed that Tau2 =4.41, Chi2 =122.58, df =8, I2=93%>50%, and P<0.01. Therefore, the random effects model was adopted for meta-analysis. The horizontal line of the 95% CI of all studies was to the left of the invalid vertical line. Meta-analysis results showed that the length of stay in the experimental group was remarkably lower than that in the control group. The MD was −7.29, 95% CI: (−8.76 to −5.82), with statistically considerable differences (Z=9.74, P<0.01) (Figure 7). The software Rev Man 5.3 was used to create a funnel plot of intraoperative bleeding volume (Figure 8). It is obvious that the circle and the midline of some studies are basically symmetrical, suggesting that the research accuracy was high and there was no publication bias.
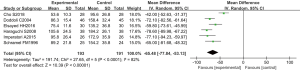
Contrast of intraoperative bleeding volume
The intraoperative bleeding volume was analyzed in 6 RCTs. There were 384 cases in total, 193 cases in the experimental group, and 191 cases in the control group. The overall heterogeneity test showed that Tau2 =191.74, Chi2 =27.65, df =5, I2=82%>50%, and P<0.01. Therefore, the random effects model was adopted for meta-analysis. The horizontal line of the 95% CI of all studies was to the left of the invalid vertical line. Meta-analysis results showed that the bleeding volume of participants in the experimental group was remarkably lower compared to that of the control group. The MD was −65.48, 95% CI: (−77.84 to −53.13), with statistically considerable differences (Z=10.39, P<0.01 (Figure 9).
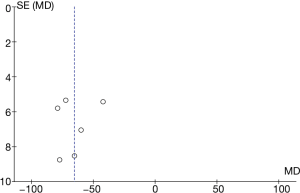
The software Rev Man 5.3 was used to create a funnel plot of intraoperative bleeding volume (Figure 10). It is obvious that the circle and the midline of some studies are basically symmetrical, suggesting that the research accuracy was high and there was no publication bias.
Contrast of complication rates
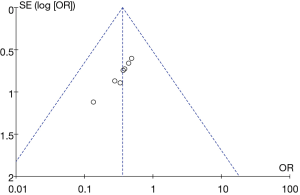
The incidence of complications was analyzed in 8 RCTs. There were 522 cases in total, 262 in the experimental group, and 260 in the control group. The overall heterogeneity test showed that Chi2 =1.07 df =7, I2=0%<50%, P=0.99>0.01. Therefore, the fixed effects model was adopted for meta-analysis. The horizontal line of the 95% CI of all studies intersects the left side of the invalid vertical line. Meta-analysis results showed that the complication rate of the experimental group was significantly lower than that of the control group, the OR was 0.40, 95% CI: (0.25 to 0.65), and the difference was substantial and considerable (Z=3.69, P=0.0002<0.01) (Figure 11).

Funnel plot of complications was visualized using Rev Man 5.3 (Figure 12). It is obvious that the circles and the midline of some studies are basically symmetrical, suggesting that the research accuracy was high and there was no publication bias.
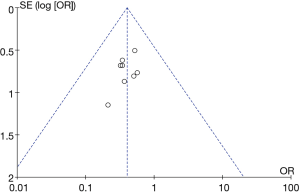
Contrast of recurrence rate
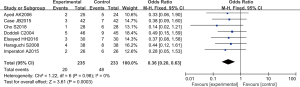
The recurrence rate was analyzed in 7 RCTs. There were 468 cases in total, 235 in the experimental group, and 233 in the control group. The overall heterogeneity test showed that Chi2 =1.22, df =6, I2=0%<50%, and P=0.98>0.01. Therefore, the fixed effects model was adopted for meta-analysis. The horizontal line of the 95% CI of all literatures intersected the left side of the invalid vertical line. Meta-analysis results showed that the recurrence rate of the experimental group was lower than that of the control group, the OR was 0.36, 95% CI: (0.20 to 0.63), and the difference was substantial and considerable (Z=3.61, P=0.0003) (Figure 13).
A funnel plot of recurrence rates was created using Rev Man 5.3 (Figure 14). It is obvious that the circle and the midline of some studies are basically symmetrical, suggesting that the research accuracy was high and there was no publication bias.
Contrast of chest tube removal time
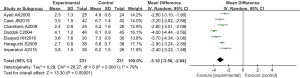
The chest tube removal time was analyzed in 7 RCTs. The total number of cases was 462, and the experimental group and the control group each had 231 cases. The overall heterogeneity test showed that Tau2 =0.29, Chi2 =28.27, df =6, I2=79%>50%, and P<0.01. Therefore, the random effects model was adopted for meta-analysis. The horizontal line of the 95% CI of all studies was to the left of the invalid vertical line. Meta-analysis results showed that the chest tube removal time for participants in the experimental group was substantially lower compared to that of the control group. The MD was −3.10, 95% CI: (−3.56 to −2.64), with statistically significant differences (Z=13.30, P<0.01) (Figure 15).
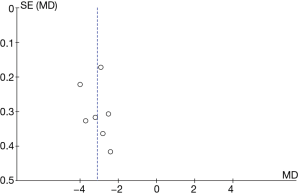
A funnel plot for chest tube removal time was created using Rev Man 5.3 (Figure 16). It is obvious that the circle and the midline of some studies are basically symmetrical, suggesting that the research accuracy was high and there was no publication bias.
Discussion
Spontaneous pneumothorax is caused by obstruction of the bronchial valve, which prevents the normal flow of gas, leading to gas retention, swelling of the bullae, and rupture to a certain extent. The VATS procedure is minimally invasive, with small wounds, short operation time, and quick recovery time (19). This study conducted meta-analysis to evaluate the efficacy of VATS. The quality classification results showed that there were 7 studies with a score of 6 or more, 3 with a score of 3–5, and 2 with a score of 2 or less. Among the 12 RCTs, 3 described the correct random allocation method, and only 2 described the correct random allocation method and described the concealment of the allocation plan in detail.
In this work, the operation time, hospital stay, complication rate, intraoperative blood loss, recurrence rate, and chest tube removal time were analyzed and compared between the VATS group and the non-VATS group. The results showed that the operation time of the experimental group was significantly inferior to that of the control group, the MD was −31.02, 95% CI was (−36.07, −25.97), Z=12.03, P<0.01. It showed that VATS treatment of spontaneous pneumothorax can greatly shorten the time during the operation and improved safety. The length of hospital stay in the experimental group was notably lower than that in the control group. The MD was −7.29, the 95% CI was (−8.76, −5.82), Z=9.74, P<0.01. The intraoperative blood loss of the experimental group was obviously inferior to that of the control group. The MD was −65.48, 95% CI was (−77.84, −53.13), Z=10.39, P<0.01. It showed that the VATS treatment of spontaneous pneumothorax can reduce the trauma to the patient, speeded up the recovery of the patient, and reduced the risk of occurrence. The complication rate of the experimental group was dramatically lower than that of the control group. The OR was 0.40, 95% CI was (0.25, 0.65), Z=3.69, P=0.0002<0.01. The recurrence rate of the experimental group was inferior to that of the control group, the OR was 0.36, and the 95% CI was (0.20, 0.63), Z=3.61, P=0.0003<0.01.
The data generated through meta-analysis showed that the effect of VATS in the treatment of spontaneous pneumothorax is better than that of other treatments, which is consistent with the study of Elsayed et al. [2016] (20). The software Rev Man 5.3 was used to create a funnel chart of each outcome indicator, showing that the circles in some studies are basically symmetrical with the midline, which indicated that the research accuracy is high and there is no bias in publication.
Conclusions
In this study, in order to explore the efficacy of VATS through meta-analysis, a composite logic search method was adopted to retrieve 12 studies which used VATS for spontaneous pneumothorax as the trial group. The meta-analysis confirmed that VATS for spontaneous pneumothorax is superior to other surgical methods. The limitations of this study were time constraints and limited literature resources. Literature retrieval may have been incomplete, and some literature may have been missing. In addition, the data of these studies were not included in this systematic review, which may have affected the results, and the included literature sample size was relatively small. Therefore, it is necessary to expand the sample size to include more recent clinical RCTs for further verification. In short, this study provides a reliable theoretical basis for the clinical treatment of spontaneous pneumothorax with VATS.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/jtd-21-652
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-21-652). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Reference
- Pogorelić Z, Gudelj R, Bjelanović D, et al. Management of the Pediatric Spontaneous Pneumothorax: The Role of Video-Assisted Thoracoscopic Surgery. J Laparoendosc Adv Surg Tech A 2020;30:569-75. [Crossref] [PubMed]
- Bricelj K, Srpčič M, Ražem A, et al. Catamenial pneumothorax since introduction of video-assisted thoracoscopic surgery: A systematic review. Wien Klin Wochenschr 2017;129:717-26. [Crossref] [PubMed]
- Chen PH, Hung WT, Chen JS. Nonintubated Video-Assisted Thoracic Surgery for the Management of Primary and Secondary Spontaneous Pneumothorax. Thorac Surg Clin 2020;30:15-24. [Crossref] [PubMed]
- Vuong NL, Elshafay A, Thao LP, et al. Efficacy of treatments in primary spontaneous pneumothorax: A systematic review and network Meta-analysis of randomized clinical trials. Respir Med 2018;137:152-66. [Crossref] [PubMed]
- Schnell J, Beer M, Eggeling S, et al. Management of Spontaneous Pneumothorax and Post-Interventional Pneumothorax: German S3 Guideline. Respiration 2019;97:370-402. [Crossref] [PubMed]
- MacDuff A, Arnold A, Harvey J, et al. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii18-31. [Crossref] [PubMed]
- Yamanaka S, Kurihara M, Watanabe K. A novel dual-covering method in video-assisted thoracic surgery for pediatric primary spontaneous pneumothorax. Surg Today 2019;49:587-92. [Crossref] [PubMed]
- Kavurmaci Ö, Akcam TI, Ergonul AG, et al. Prolonged air leak after video-assisted thoracoscopic surgery in patients with primary spontaneous pneumothorax. Niger J Clin Pract 2019;22:1292-7. [Crossref] [PubMed]
- Schramel FM, Sutedja TG, Braber JC, et al. Cost-effectiveness of video-assisted thoracoscopic surgery versus conservative treatment for first time or recurrent spontaneous pneumothorax. Eur Respir J 1996;9:1821-5. [Crossref] [PubMed]
- Haraguchi S, Koizumi K, Hioki M, et al. Postoperative recurrences of pneumothorax in video-assisted thoracoscopic surgery for primary spontaneous pneumothorax in young patients. J Nippon Med Sch 2008;75:91-5. [Crossref] [PubMed]
- Chambers A, Scarci M. In patients with first-episode primary spontaneous pneumothorax is video-assisted thoracoscopic surgery superior to tube thoracostomy alone in terms of time to resolution of pneumothorax and incidence of recurrence? Interact Cardiovasc Thorac Surg 2009;9:1003-8. [Crossref] [PubMed]
- Case JB, Mayhew PD, Singh A. Evaluation of Video-Assisted Thoracic Surgery for Treatment of Spontaneous Pneumothorax and Pulmonary Bullae in Dogs. Vet Surg 2015;44:31-8. [Crossref] [PubMed]
- Imperatori A, Rotolo N, Spagnoletti M, et al. Risk factors for postoperative recurrence of spontaneous pneumothorax treated by video-assisted thoracoscopic surgery†. Interact Cardiovasc Thorac Surg 2015;20:647-51; discussion 651-2. [Crossref] [PubMed]
- Ayed AK, Chandrasekaran C, Sukumar M. Video-assisted thoracoscopic surgery for primary spontaneous pneumothorax: clinicopathological correlation. Eur J Cardiothorac Surg 2006;29:221-5. [Crossref] [PubMed]
- Shaikhrezai K, Thompson AI, Parkin C, et al. Video-assisted thoracoscopic surgery management of spontaneous pneumothorax--long-term results. Eur J Cardiothorac Surg 2011;40:120-3. [Crossref] [PubMed]
- Doddoli C, Barlési F, Fraticelli A, et al. Video-assisted thoracoscopic management of recurrent primary spontaneous pneumothorax after prior talc pleurodesis: a feasible, safe and efficient treatment option. Eur J Cardiothorac Surg 2004;26:889-92. [Crossref] [PubMed]
- Cho S, Jheon S, Kim DK, et al. Results of repeated video-assisted thoracic surgery for recurrent pneumothorax after primary spontaneous pneumothorax. Eur J Cardiothorac Surg 2018;53:857-61. [Crossref] [PubMed]
- Chen L, Liu F, Wang B, et al. Subxiphoid vs transthoracic approach thoracoscopic surgery for spontaneous pneumothorax: a propensity score-matched analysis. BMC Surg 2019;19:46. [Crossref] [PubMed]
- Williams K, Lautz TB, Leon AH, et al. Optimal timing of video-assisted thoracoscopic surgery for primary spontaneous pneumothorax in children. J Pediatr Surg 2018;53:1858-61. [Crossref] [PubMed]
- Elsayed HH, Hassaballa A, Ahmed T. Is video-assisted thoracoscopic surgery talc pleurodesis superior to talc pleurodesis via tube thoracostomy in patients with secondary spontaneous pneumothorax? Interact Cardiovasc Thorac Surg 2016;23:459-61. [Crossref] [PubMed]
(English Language Editor: J. Jones)